Actin cortex architecture regulates cell surface tension
- PMID: 28530659
- PMCID: PMC5536221
- DOI: 10.1038/ncb3525
Actin cortex architecture regulates cell surface tension
Abstract
Animal cell shape is largely determined by the cortex, a thin actin network underlying the plasma membrane in which myosin-driven stresses generate contractile tension. Tension gradients result in local contractions and drive cell deformations. Previous cortical tension regulation studies have focused on myosin motors. Here, we show that cortical actin network architecture is equally important. First, we observe that actin cortex thickness and tension are inversely correlated during cell-cycle progression. We then show that the actin filament length regulators CFL1, CAPZB and DIAPH1 regulate mitotic cortex thickness and find that both increasing and decreasing thickness decreases tension in mitosis. This suggests that the mitotic cortex is poised close to a tension maximum. Finally, using a computational model, we identify a physical mechanism by which maximum tension is achieved at intermediate actin filament lengths. Our results indicate that actin network architecture, alongside myosin activity, is key to cell surface tension regulation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
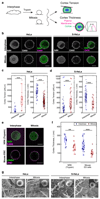


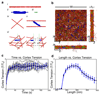
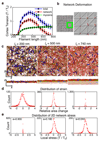

References
-
- Salbreux G, Charras G, Paluch E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012;22:536–545. - PubMed
-
- Bray D, White JG. Cortical flow in animal cells. Science. 1988;239:883–888. - PubMed
-
- Clark AG, Paluch E. Mechanics and regulation of cell shape during the cell cycle. In: Kubiak JZ, editor. Cell Cycle in Development. Vol. 1. Springer-Verlag; Berlin, Heidelberg, Germany: 2011. pp. 31–73. - PubMed
-
- Heisenberg CP, Bellaiche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. - PubMed
-
- Levayer R, Lecuit T. Biomechanical regulation of contractility: spatial control and dynamics. Trends Cell Biol. 2012;22:61–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

