Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications-Focus on Prostate and Breast Cancer
- PMID: 28531102
- PMCID: PMC5455010
- DOI: 10.3390/ijms18051102
Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications-Focus on Prostate and Breast Cancer
Abstract
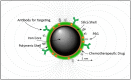
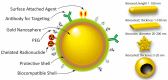
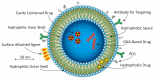
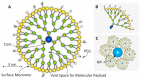
Prostate and breast cancer are the second most and most commonly diagnosed cancer in men and women worldwide, respectively. The American Cancer Society estimates that during 2016 in the USA around 430,000 individuals were diagnosed with one of these two types of cancers, and approximately 15% of them will die from the disease. In Europe, the rate of incidences and deaths are similar to those in the USA. Several different more or less successful diagnostic and therapeutic approaches have been developed and evaluated in order to tackle this issue and thereby decrease the death rates. By using nanoparticles as vehicles carrying both diagnostic and therapeutic molecular entities, individualized targeted theranostic nanomedicine has emerged as a promising option to increase the sensitivity and the specificity during diagnosis, as well as the likelihood of survival or prolonged survival after therapy. This article presents and discusses important and promising different kinds of nanoparticles, as well as imaging and therapy options, suitable for theranostic applications. The presentation of different nanoparticles and theranostic applications is quite general, but there is a special focus on prostate cancer. Some references and aspects regarding breast cancer are however also presented and discussed. Finally, the prostate cancer case is presented in more detail regarding diagnosis, staging, recurrence, metastases, and treatment options available today, followed by possible ways to move forward applying theranostics for both prostate and breast cancer based on promising experiments performed until today.
Keywords: breast cancer; nanomedicine; nanoparticles; prostate cancer; theranostics.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Cancer Theranostic Applications of Albumin-Coated Tobacco Mosaic Virus Nanoparticles.ACS Appl Mater Interfaces. 2018 Nov 21;10(46):39468-39477. doi: 10.1021/acsami.8b12499. Epub 2018 Nov 7. ACS Appl Mater Interfaces. 2018. PMID: 30403330 Free PMC article.
-
Prospects of nano-theranostic approaches against breast and cervical cancer.Biochim Biophys Acta Rev Cancer. 2024 Nov;1879(6):189227. doi: 10.1016/j.bbcan.2024.189227. Epub 2024 Nov 28. Biochim Biophys Acta Rev Cancer. 2024. PMID: 39612962 Review.
-
Prostate Cancer Imaging and Therapy: Potential Role of Nanoparticles.J Nucl Med. 2016 Oct;57(Suppl 3):105S-110S. doi: 10.2967/jnumed.115.170738. J Nucl Med. 2016. PMID: 27694162 Review.
-
Biomimetic Targeted Theranostic Nanoparticles for Breast Cancer Treatment.Molecules. 2022 Oct 1;27(19):6473. doi: 10.3390/molecules27196473. Molecules. 2022. PMID: 36235009 Free PMC article.
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
Cited by
-
Radioiodination of Modified Porous Silica Nanoparticles as a Potential Candidate of Iodine-131 Drugs Vehicle.ACS Omega. 2022 Apr 18;7(16):13494-13506. doi: 10.1021/acsomega.1c06492. eCollection 2022 Apr 26. ACS Omega. 2022. PMID: 35559138 Free PMC article.
-
Application of nanotechnology in the diagnosis and treatment of bladder cancer.J Nanobiotechnology. 2021 Nov 27;19(1):393. doi: 10.1186/s12951-021-01104-y. J Nanobiotechnology. 2021. PMID: 34838048 Free PMC article. Review.
-
Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies.Int J Environ Res Public Health. 2020 Mar 20;17(6):2078. doi: 10.3390/ijerph17062078. Int J Environ Res Public Health. 2020. PMID: 32245065 Free PMC article. Review.
-
Nucleic Acid Aptamers: Emerging Applications in Medical Imaging, Nanotechnology, Neurosciences, and Drug Delivery.Int J Mol Sci. 2017 Nov 16;18(11):2430. doi: 10.3390/ijms18112430. Int J Mol Sci. 2017. PMID: 29144411 Free PMC article. Review.
-
Nanoparticle-Based Radioconjugates for Targeted Imaging and Therapy of Prostate Cancer.Molecules. 2023 May 16;28(10):4122. doi: 10.3390/molecules28104122. Molecules. 2023. PMID: 37241862 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

