Defining the vaccination window for respiratory syncytial virus (RSV) using age-seroprevalence data for children in Kilifi, Kenya
- PMID: 28531224
- PMCID: PMC5439681
- DOI: 10.1371/journal.pone.0177803
Defining the vaccination window for respiratory syncytial virus (RSV) using age-seroprevalence data for children in Kilifi, Kenya
Abstract
Background: Respiratory syncytial virus (RSV) is an important cause of lower respiratory tract disease in early life and a target for vaccine prevention. Data on the age-prevalence of RSV specific antibodies will inform on optimizing vaccine delivery.
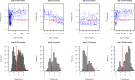
Methods: Archived plasma samples were randomly selected within age strata from 960 children less than 145 months of age admitted to Kilifi County Hospital pediatric wards between 2007 and 2010. Samples were tested for antibodies to RSV using crude virus IgG ELISA. Seroprevalence (and 95% confidence intervals) was estimated as the proportion of children with specific antibodies above a defined cut-off level. Nested catalytic models were used to explore different assumptions on antibody dynamics and estimate the rates of decay of RSV specific maternal antibody and acquisition of infection with age, and the average age of infection.
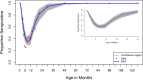
Results: RSV specific antibody prevalence was 100% at age 0-<1month, declining rapidly over the first 6 months of life, followed by an increase in the second half of the first year of life and beyond. Seroprevalence was lowest throughout the age range 5-11 months; all children were seropositive beyond 3 years of age. The best fit model to the data yielded estimates for the rate of infection of 0.78/person/year (95% CI 0.65-0.97) and 1.69/person/year (95% CI 1.27-2.04) for ages 0-<1 year and 1-<12 years, respectively. The rate of loss of maternal antibodies was estimated as 2.54/year (95% CI 2.30-2.90), i.e. mean duration 4.7 months. The mean age at primary infection was estimated at 15 months (95% CI 13-18).
Conclusions: The rate of decay of maternal antibody prevalence and subsequent age-acquisition of infection are rapid, and the average age at primary infection early. The vaccination window is narrow, and suggests optimal targeting of vaccine to infants 5 months and above to achieve high seroconversion.
Conflict of interest statement
Figures



References
-
- Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013; 381(9875):1380–90. doi: 10.1016/S0140-6736(12)61901-1 - DOI - PMC - PubMed
-
- Lee MS, Walker RE, Mendelman PM. Medical burden of respiratory syncytial virus and parainfluenza virus type 3 infection among US children. Implications for design of vaccine trials. Hum Vaccin. 2005; 1(1):6–11. - PubMed
-
- Cooney MK, Fox JP, Hall CE. The Seattle Virus Watch. VI. Observations of infections with and illness due to parainfluenza, mumps and respiratory syncytial viruses and Mycoplasma pneumoniae. Am J Epidemiol. 1975; 101(6):532–51. - PubMed
-
- Noyola DE, Arteaga-Dominguez G. Contribution of respiratory syncytial virus, influenza and parainfluenza viruses to acute respiratory infections in San Luis Potosi, Mexico. Pediatr Infect Dis J. 2005; 24(12):1049–52. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

