Correlation of immune phenotype with IDH mutation in diffuse glioma
- PMID: 28531337
- PMCID: PMC5737620
- DOI: 10.1093/neuonc/nox054
Correlation of immune phenotype with IDH mutation in diffuse glioma
Abstract
Background: Tumor infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) are targets of immune checkpoint inhibitors.
Methods: Forty-three World Health Organization (WHO) grade II/III gliomas (39 IDH-mutant [mut], 4 IDH-wildtype [wt]) and 14 IDH-mut glioblastomas (GBM) were analyzed for TIL (CD3+; PD1+) infiltration and PD-L1 expression. Results were compared with the data of a previously published series of 117 IDH-wt glioblastomas. PD-L1 gene expression levels were evaluated in 677 diffuse gliomas grades II-IV from The Cancer Genome Atlas (TCGA) database.
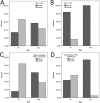
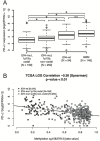
Results: TIL and PD-L1 expression were observed in approximately half of WHO grade II/III gliomas. IDH-wt status was associated with significantly higher TIL infiltration and PD-L1 expression among all (grades II-IV) cases (n = 174, P < 0.001) and within the cohort of glioblastomas (n = 131, P < 0.001). In low-grade glioma (LGG) and glioblastoma cohorts of TCGA, significantly higher PD-L1 gene expression levels were evident in IDH-wt compared with IDH-mut samples (LGG: N = 516; P = 1.933e-11, GBM: N = 161; P < 0.009). Lower PD-L1 gene expression was associated with increased promoter methylation (Spearman correlation coefficient -0.36; P < 0.01) in the LGG cohort of TCGA. IDH-mut gliomas had higher PD-L1 gene promoter methylation levels than IDH-wt gliomas (P < 0.01).
Conclusions: The immunological tumor microenvironment of diffuse gliomas differs in association with IDH mutation status. IDH-wt gliomas display a more prominent TIL infiltration and higher PD-L1 expression than IDH-mut cases. Mechanistically this may be at least in part due to differential PD-L1 gene promoter methylation levels. Our findings may be relevant for immune modulatory treatment strategies in glioma patients.
Keywords: IDH mutation; PD-L1; glioblastoma; immune microenvironment; low grade glioma; promoter methylation.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures

Comment in
-
ID(H)entifying checkpoint inhibitor candidates among diffuse glioma.Neuro Oncol. 2017 Oct 19;19(11):1427-1428. doi: 10.1093/neuonc/nox145. Neuro Oncol. 2017. PMID: 29036631 Free PMC article. No abstract available.
References
-
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. - PubMed
-
- Reuss DE, Kratz A, Sahm F, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015;130(3):407–417. - PubMed
-
- Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7467. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

