Functional Circuitry of the Retina
- PMID: 28532365
- PMCID: PMC5749398
- DOI: 10.1146/annurev-vision-082114-035334
Functional Circuitry of the Retina
Abstract
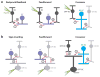
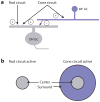
The mammalian retina is an important model system for studying neural circuitry: Its role in sensation is clear, its cell types are relatively well defined, and its responses to natural stimuli-light patterns-can be studied in vitro. To solve the retina, we need to understand how the circuits presynaptic to its output neurons, ganglion cells, divide the visual scene into parallel representations to be assembled and interpreted by the brain. This requires identifying the component interneurons and understanding how their intrinsic properties and synapses generate circuit behaviors. Because the cellular composition and fundamental properties of the retina are shared across species, basic mechanisms studied in the genetically modifiable mouse retina apply to primate vision. We propose that the apparent complexity of retinal computation derives from a straightforward mechanism-a dynamic balance of synaptic excitation and inhibition regulated by use-dependent synaptic depression-applied differentially to the parallel pathways that feed ganglion cells.
Keywords: adaptation; amacrine cell; bipolar cell; ganglion cell; parallel pathways; synaptic depression.
Figures
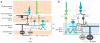



References
-
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–23. - PubMed
-
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–19. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

