Serum amyloid A: an ozone-induced circulating factor with potentially important functions in the lung-brain axis
- PMID: 28533327
- PMCID: PMC5572691
- DOI: 10.1096/fj.201600857RRR
Serum amyloid A: an ozone-induced circulating factor with potentially important functions in the lung-brain axis
Erratum in
-
Erratum.FASEB J. 2018 Jan;32(1):535. doi: 10.1096/fj.201600857RRRERR. FASEB J. 2018. PMID: 29295887 Free PMC article. No abstract available.
Abstract
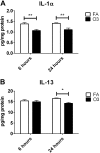
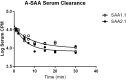
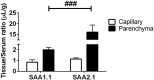
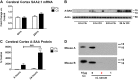
Accumulating evidence suggests that O3 exposure may contribute to CNS dysfunction. Here, we posit that inflammatory and acute-phase proteins in the circulation increase after O3 exposure and systemically convey signals of O3 exposure to the CNS. To model acute O3 exposure, female Balb/c mice were exposed to 3 ppm O3 or forced air for 2 h and were studied after 6 or 24 h. Of 23 cytokines and chemokines, only KC/CXCL1 was increased in blood 6 h after O3 exposure. The acute-phase protein serum amyloid A (A-SAA) was significantly increased by 24 h, whereas C-reactive protein was unchanged. A-SAA in blood correlated with total leukocytes, macrophages, and neutrophils in bronchoalveolar lavage from O3-exposed mice. A-SAA mRNA and protein were increased in the liver. We found that both isoforms of A-SAA completely crossed the intact blood-brain barrier, although the rate of SAA2.1 influx was approximately 5 times faster than that of SAA1.1. Finally, A-SAA protein, but not mRNA, was increased in the CNS 24 h post-O3 exposure. Our findings suggest that A-SAA is functionally linked to pulmonary inflammation in our O3 exposure model and that A-SAA could be an important systemic signal of O3 exposure to the CNS.-Erickson, M. A., Jude, J., Zhao, H., Rhea, E. M., Salameh, T. S., Jester, W., Pu, S., Harrowitz, J., Nguyen, N., Banks, W. A., Panettieri, R. A., Jr., Jordan-Sciutto, K. L. Serum amyloid A: an ozone-induced circulating factor with potentially important functions in the lung-brain axis.
Keywords: acute-phase proteins; air pollution; blood-brain barrier; cytokines; microglia.
© FASEB.
Conflict of interest statement
This work was supported by the U. S. National Institutes of Health (NIH) National Institute of Environmental Health Sciences (Grants T32ES019851 to M.A.E. and J.J., and F32ES025076 to M.A.E.), NIH National Institute of Neurological Disorders and Stroke (Grant R21NS093368 to W.A.B. and T.S.S.), NIH National Institute on Aging (Grant T32AG000057 to E.M.R.); and NIH National Institute of Mental Health (Grants R01MH106967 to K.L.J.-S. and P30ES013508 to R.A.P.).
Figures





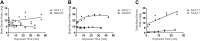
References
-
- Lelieveld J., Evans J. S., Fnais M., Giannadaki D., and Pozzer A. (2015) The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525, 367–371 - PubMed
-
- Chen J. C., and Schwartz J. (2009) Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology 30, 231–239 - PubMed
-
- Jung C. R., Lin Y. T., and Hwang B. F. (2015) Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J. Alzheimers Dis. 44, 573–584 - PubMed
-
- Montresor-López J. A., Yanosky J. D., Mittleman M. A., Sapkota A., He X., Hibbert J. D., Wirth M. D., and Puett R. C. (2016) Short-term exposure to ambient ozone and stroke hospital admission: a case-crossover analysis. J. Expo. Sci. Environ. Epidemiol. 26, 162–166 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

