Chronic Stimulation of Renin Cells Leads to Vascular Pathology
- PMID: 28533331
- PMCID: PMC5501939
- DOI: 10.1161/HYPERTENSIONAHA.117.09283
Chronic Stimulation of Renin Cells Leads to Vascular Pathology
Abstract
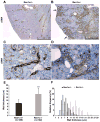
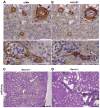
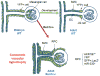
Experimental or spontaneous genomic mutations of the renin-angiotensin system or its pharmacological inhibition in early life leads to renal abnormalities, including poorly developed renal medulla, papillary atrophy, hydronephrosis, inability to concentrate the urine, polyuria, polydipsia, renal failure, and anemia. At the core of such complex phenotype is the presence of unique vascular abnormalities: the renal arterioles do not branch or elongate properly and they have disorganized, concentric hypertrophy. This lesion has been puzzling because it is often found in hypertensive individuals whereas mutant or pharmacologically inhibited animals are hypotensive. Remarkably, when renin cells are ablated with diphtheria toxin, the vascular hypertrophy does not occur, suggesting that renin cells per se may contribute to the vascular disease. To test this hypothesis, on a Ren1c-/- background, we generated mutant mice with reporter expression (Ren1c-/-;Ren1c-Cre;R26R.mTmG and Ren1c-/-;Ren1c-Cre;R26R.LacZ) to trace the fate of reninnull cells. To assess whether reninnull cells maintain their renin promoter active, we used Ren1c-/-;Ren1c-YFP mice that transcribe YFP (yellow fluorescent protein) directed by the renin promoter. We also followed the expression of Akr1b7 and miR-330-5p, markers of cells programmed for the renin phenotype. Contrary to what we expected, reninnull cells did not die or disappear. Instead, they survived, increased in number along the renal arterial tree, and maintained an active molecular memory of the myoepitheliod renin phenotype. Furthermore, null cells of the renin lineage occupied the walls of the arteries and arterioles in a chaotic, directionless pattern directly contributing to the concentric arterial hypertrophy.
Keywords: kidney; mice; mutation; phenotype; renin-angiotensin system.
© 2017 American Heart Association, Inc.
Conflict of interest statement
None
Figures


Comment in
-
Of Mice and Renin.Hypertension. 2017 Jul;70(1):35-37. doi: 10.1161/HYPERTENSIONAHA.117.09379. Epub 2017 May 22. Hypertension. 2017. PMID: 28533332 No abstract available.
References
-
- Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. - PubMed
-
- Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, Fukamizu A, Murakami K. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269:31334–31337. - PubMed
-
- Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O. Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem. 1999;274:14210–14217. - PubMed
-
- Okubo S, Niimura F, Matsusaka T, Fogo A, Hogan BL, Ichikawa I. Angiotensinogen gene null-mutant mice lack homeostatic regulation of glomerular filtration and tubular reabsorption. Kidney Int. 1998;53:617–625. - PubMed
-
- Nagata M, Tanimoto K, Fukamizu A, Kon Y, Sugiyama F, Yagami K, Murakami K, Watanabe T. Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest. 1996;75:745–753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

