A Coding Variant in the Gene Bardet-Biedl Syndrome 4 (BBS4) Is Associated with a Novel Form of Canine Progressive Retinal Atrophy
- PMID: 28533336
- PMCID: PMC5499139
- DOI: 10.1534/g3.117.043109
A Coding Variant in the Gene Bardet-Biedl Syndrome 4 (BBS4) Is Associated with a Novel Form of Canine Progressive Retinal Atrophy
Abstract
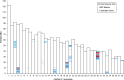
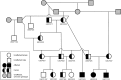
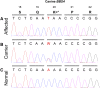
Progressive retinal atrophy is a common cause of blindness in the dog and affects >100 breeds. It is characterized by gradual vision loss that occurs due to the degeneration of photoreceptor cells in the retina. Similar to the human counterpart retinitis pigmentosa, the canine disorder is clinically and genetically heterogeneous and the underlying cause remains unknown for many cases. We use a positional candidate gene approach to identify putative variants in the Hungarian Puli breed using genotyping data of 14 family-based samples (CanineHD BeadChip array, Illumina) and whole-genome sequencing data of two proband and two parental samples (Illumina HiSeq 2000). A single nonsense SNP in exon 2 of BBS4 (c.58A > T, p.Lys20*) was identified following filtering of high quality variants. This allele is highly associated (PCHISQ = 3.425e-14, n = 103) and segregates perfectly with progressive retinal atrophy in the Hungarian Puli. In humans, BBS4 is known to cause Bardet-Biedl syndrome which includes a retinitis pigmentosa phenotype. From the observed coding change we expect that no functional BBS4 can be produced in the affected dogs. We identified canine phenotypes comparable with Bbs4-null mice including obesity and spermatozoa flagella defects. Knockout mice fail to form spermatozoa flagella. In the affected Hungarian Puli spermatozoa flagella are present, however a large proportion of sperm are morphologically abnormal and <5% are motile. This suggests that BBS4 contributes to flagella motility but not formation in the dog. Our results suggest a promising opportunity for studying Bardet-Biedl syndrome in a large animal model.
Keywords: Hungarian Puli; blindness; infertility; obesity; whole-genome sequencing.
Copyright © 2017 Chew et al.
Figures

References
-
- Acland G. M., Ray K., Mellersh C. S., Langston A. A., Rine J., et al. , 1999. A novel retinal degeneration locus identified by linkage and comparative mapping of canine early retinal degeneration. Genomics 59: 134–142. - PubMed
-
- Acland G. M., Aguirre G. D., Ray J., Zhang Q., Aleman T. S., et al. , 2001. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 28: 92–95. - PubMed
-
- Axelsson E., Ratnakumar A., Arendt M. L., Maqbool K., Webster M. T., et al. , 2013. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495: 360–364. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
