Phosphorylated Presenilin 1 decreases β-amyloid by facilitating autophagosome-lysosome fusion
- PMID: 28533369
- PMCID: PMC5502640
- DOI: 10.1073/pnas.1705240114
Phosphorylated Presenilin 1 decreases β-amyloid by facilitating autophagosome-lysosome fusion
Abstract
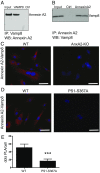
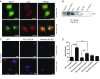
Presenilin 1 (PS1), the catalytic subunit of the γ-secretase complex, cleaves βCTF to produce Aβ. We have shown that PS1 regulates Aβ levels by a unique bifunctional mechanism. In addition to its known role as the catalytic subunit of the γ-secretase complex, selective phosphorylation of PS1 on Ser367 decreases Aβ levels by increasing βCTF degradation through autophagy. Here, we report the molecular mechanism by which PS1 modulates βCTF degradation. We show that PS1 phosphorylated at Ser367, but not nonphosphorylated PS1, interacts with Annexin A2, which, in turn, interacts with the lysosomal N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) Vamp8. Annexin A2 facilitates the binding of Vamp8 to the autophagosomal SNARE Syntaxin 17 to modulate the fusion of autophagosomes with lysosomes. Thus, PS1 phosphorylated at Ser367 has an antiamyloidogenic function, promoting autophagosome-lysosome fusion and increasing βCTF degradation. Drugs designed to increase the level of PS1 phosphorylated at Ser367 should be useful in the treatment of Alzheimer's disease.
Keywords: Annexin A2; Presenilin 1; autophagosome–lysosome fusion; autophagy; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
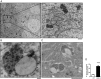
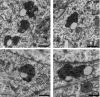
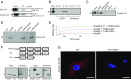
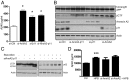
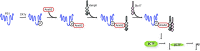
Comment in
-
Versatility of presenilin 1.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):6885-6887. doi: 10.1073/pnas.1707809114. Epub 2017 Jun 23. Proc Natl Acad Sci U S A. 2017. PMID: 28645897 Free PMC article. No abstract available.
Similar articles
-
Amyloidogenic and anti-amyloidogenic properties of presenilin 1.Adv Pharmacol. 2021;90:239-251. doi: 10.1016/bs.apha.2020.09.010. Epub 2020 Oct 24. Adv Pharmacol. 2021. PMID: 33706935 Review.
-
Bidirectional regulation of Aβ levels by Presenilin 1.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):7142-7147. doi: 10.1073/pnas.1705235114. Epub 2017 May 22. Proc Natl Acad Sci U S A. 2017. PMID: 28533411 Free PMC article.
-
The macrophage-specific V-ATPase subunit ATP6V0D2 restricts inflammasome activation and bacterial infection by facilitating autophagosome-lysosome fusion.Autophagy. 2019 Jun;15(6):960-975. doi: 10.1080/15548627.2019.1569916. Epub 2019 Jan 29. Autophagy. 2019. PMID: 30681394 Free PMC article.
-
mTOR-mediated phosphorylation of VAMP8 and SCFD1 regulates autophagosome maturation.Nat Commun. 2021 Nov 16;12(1):6622. doi: 10.1038/s41467-021-26824-5. Nat Commun. 2021. PMID: 34785650 Free PMC article.
-
New insights regarding SNARE proteins in autophagosome-lysosome fusion.Autophagy. 2021 Oct;17(10):2680-2688. doi: 10.1080/15548627.2020.1823124. Epub 2020 Sep 24. Autophagy. 2021. PMID: 32924745 Free PMC article. Review.
Cited by
-
Is γ-secretase a beneficial inactivating enzyme of the toxic APP C-terminal fragment C99?J Biol Chem. 2021 Jan-Jun;296:100489. doi: 10.1016/j.jbc.2021.100489. Epub 2021 Mar 1. J Biol Chem. 2021. PMID: 33662398 Free PMC article. Review.
-
[Progress on loss-of-function hypothesis of presenilin-1 mutations in Alzheimer diseases].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020 Aug 25;49(4):487-499. doi: 10.3785/j.issn.1008-9292.2020.08.03. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 32985163 Free PMC article. Chinese.
-
Role of SNAREs in Neurodegenerative Diseases.Cells. 2021 Apr 23;10(5):991. doi: 10.3390/cells10050991. Cells. 2021. PMID: 33922505 Free PMC article. Review.
-
Exploring the bi-directional relationship between autophagy and Alzheimer's disease.CNS Neurosci Ther. 2020 Feb;26(2):155-166. doi: 10.1111/cns.13216. Epub 2019 Sep 10. CNS Neurosci Ther. 2020. PMID: 31503421 Free PMC article. Review.
-
Protein levels of ADAM10, BACE1, and PSEN1 in platelets and leukocytes of Alzheimer's disease patients.Eur Arch Psychiatry Clin Neurosci. 2019 Dec;269(8):963-972. doi: 10.1007/s00406-018-0905-3. Epub 2018 May 29. Eur Arch Psychiatry Clin Neurosci. 2019. PMID: 29845446
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

