Phosphorylated Presenilin 1 decreases β-amyloid by facilitating autophagosome-lysosome fusion
- PMID: 28533369
- PMCID: PMC5502640
- DOI: 10.1073/pnas.1705240114
Phosphorylated Presenilin 1 decreases β-amyloid by facilitating autophagosome-lysosome fusion
Abstract
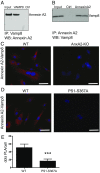
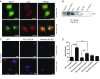
Presenilin 1 (PS1), the catalytic subunit of the γ-secretase complex, cleaves βCTF to produce Aβ. We have shown that PS1 regulates Aβ levels by a unique bifunctional mechanism. In addition to its known role as the catalytic subunit of the γ-secretase complex, selective phosphorylation of PS1 on Ser367 decreases Aβ levels by increasing βCTF degradation through autophagy. Here, we report the molecular mechanism by which PS1 modulates βCTF degradation. We show that PS1 phosphorylated at Ser367, but not nonphosphorylated PS1, interacts with Annexin A2, which, in turn, interacts with the lysosomal N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) Vamp8. Annexin A2 facilitates the binding of Vamp8 to the autophagosomal SNARE Syntaxin 17 to modulate the fusion of autophagosomes with lysosomes. Thus, PS1 phosphorylated at Ser367 has an antiamyloidogenic function, promoting autophagosome-lysosome fusion and increasing βCTF degradation. Drugs designed to increase the level of PS1 phosphorylated at Ser367 should be useful in the treatment of Alzheimer's disease.
Keywords: Annexin A2; Presenilin 1; autophagosome–lysosome fusion; autophagy; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
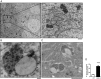
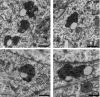
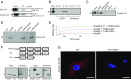
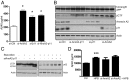
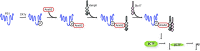
Comment in
-
Versatility of presenilin 1.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):6885-6887. doi: 10.1073/pnas.1707809114. Epub 2017 Jun 23. Proc Natl Acad Sci U S A. 2017. PMID: 28645897 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

