Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality
- PMID: 28533370
- PMCID: PMC5468619
- DOI: 10.1073/pnas.1703546114
Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality
Abstract
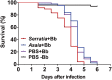
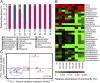
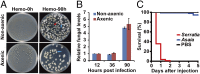
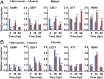
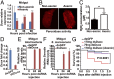
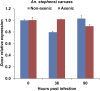
The insect gut microbiota plays crucial roles in modulating the interactions between the host and intestinal pathogens. Unlike viruses, bacteria, and parasites, which need to be ingested to cause disease, entomopathogenic fungi infect insects through the cuticle and proliferate in the hemolymph. However, interactions between the gut microbiota and entomopathogenic fungi are unknown. Here we show that the pathogenic fungus Beauveria bassiana interacts with the gut microbiota to accelerate mosquito death. After topical fungal infection, mosquitoes with gut microbiota die significantly faster than mosquitoes without microbiota. Furthermore, fungal infection causes dysbiosis of mosquito gut microbiota with a significant increase in gut bacterial load and a significant decrease in bacterial diversity. In particular, the opportunistic pathogenic bacterium Serratia marcescens overgrows in the midgut and translocates to the hemocoel, which promotes fungal killing of mosquitoes. We further reveal that fungal infection down-regulates antimicrobial peptide and dual oxidase expression in the midgut. Duox down-regulation in the midgut is mediated by secretion of the toxin oosporein from B. bassiana Our findings reveal the important contribution of the gut microbiota in B. bassiana-killing activity, providing new insights into the mechanisms of fungal pathogenesis in insects.
Keywords: Anopheles; dysbiosis; entomopathogenic fungus; gut microbiota; immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

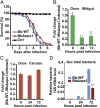
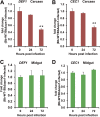

References
-
- Fan Y, Borovsky D, Hawkings C, Ortiz-Urquiza A, Keyhani NO. Exploiting host molecules to augment mycoinsecticide virulence. Nat Biotechnol. 2012;30:35–37. - PubMed
-
- Kanzok SM, Jacobs-Lorena M. Entomopathogenic fungi as biological insecticides to control malaria. Trends Parasitol. 2006;22:49–51. - PubMed
-
- Lord JC. From Metchnikoff to Monsanto and beyond: The path of microbial control. J Invertebr Pathol. 2005;89:19–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

