Solitary bees reduce investment in communication compared with their social relatives
- PMID: 28533385
- PMCID: PMC5488929
- DOI: 10.1073/pnas.1620780114
Solitary bees reduce investment in communication compared with their social relatives
Abstract
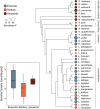
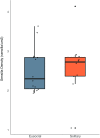
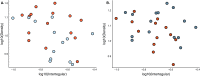
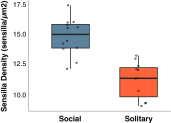
Social animals must communicate to define group membership and coordinate social organization. For social insects, communication is predominantly mediated through chemical signals, and as social complexity increases, so does the requirement for a greater diversity of signals. This relationship is particularly true for advanced eusocial insects, including ants, bees, and wasps, whose chemical communication systems have been well-characterized. However, we know surprisingly little about how these communication systems evolve during the transition between solitary and group living. Here, we demonstrate that the sensory systems associated with signal perception are evolutionarily labile. In particular, we show that differences in signal production and perception are tightly associated with changes in social behavior in halictid bees. Our results suggest that social species require a greater investment in communication than their solitary counterparts and that species that have reverted from eusociality to solitary living have repeatedly reduced investment in these potentially costly sensory perception systems.
Keywords: communication; comparative methods; halictid bees; social behavior.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Sensory and cognitive adaptations to social living in insect societies.Proc Natl Acad Sci U S A. 2017 Jun 20;114(25):6424-6426. doi: 10.1073/pnas.1707141114. Epub 2017 Jun 9. Proc Natl Acad Sci U S A. 2017. PMID: 28600351 Free PMC article. No abstract available.
References
-
- Blum MS. Semiochemical parsimony in the Arthropoda. Annu Rev Entomol. 1996;41:353–374. - PubMed
-
- Blum MS, Brand JM. Social insect pheromones: Their chemistry and function. Am Zool. 1972;12:553–576.
-
- Blum M. Pheromonal sociality in the Hymenoptera. In: Birch M, editor. Pheromones. Vol 32. North-Holland; Amsterdam: 1974. pp. 222–249.
-
- Le Conte Y, Hefetz A. Primer pheromones in social hymenoptera. Annu Rev Entomol. 2008;53:523–542. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

