Repression of phosphatidylinositol transfer protein α ameliorates the pathology of Duchenne muscular dystrophy
- PMID: 28533404
- PMCID: PMC5468635
- DOI: 10.1073/pnas.1703556114
Repression of phosphatidylinositol transfer protein α ameliorates the pathology of Duchenne muscular dystrophy
Abstract
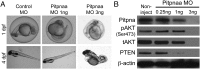
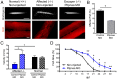
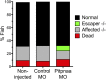
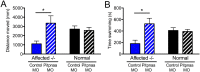
Duchenne muscular dystrophy (DMD) is a progressive muscle wasting disease caused by X-linked inherited mutations in the DYSTROPHIN (DMD) gene. Absence of dystrophin protein from the sarcolemma causes severe muscle degeneration, fibrosis, and inflammation, ultimately leading to cardiorespiratory failure and premature death. Although there are several promising strategies under investigation to restore dystrophin protein expression, there is currently no cure for DMD, and identification of genetic modifiers as potential targets represents an alternative therapeutic strategy. In a Brazilian golden retriever muscular dystrophy (GRMD) dog colony, two related dogs demonstrated strikingly mild dystrophic phenotypes compared with those typically observed in severely affected GRMD dogs despite lacking dystrophin. Microarray analysis of these "escaper" dogs revealed reduced expression of phosphatidylinositol transfer protein-α (PITPNA) in escaper versus severely affected GRMD dogs. Based on these findings, we decided to pursue investigation of modulation of PITPNA expression on dystrophic pathology in GRMD dogs, dystrophin-deficient sapje zebrafish, and human DMD myogenic cells. In GRMD dogs, decreased expression of Pitpna was associated with increased phosphorylated Akt (pAkt) expression and decreased PTEN levels. PITPNA knockdown by injection of morpholino oligonucleotides in sapje zebrafish also increased pAkt, rescued the abnormal muscle phenotype, and improved long-term sapje mutant survival. In DMD myotubes, PITPNA knockdown by lentiviral shRNA increased pAkt and increased myoblast fusion index. Overall, our findings suggest PIPTNA as a disease modifier that accords benefits to the abnormal signaling, morphology, and function of dystrophic skeletal muscle, and may be a target for DMD and related neuromuscular diseases.
Keywords: Duchenne muscular dystrophy; genetic modifier; phosphatidylinositol transfer protein-α; skeletal muscle.
Conflict of interest statement
Conflict of interest statement: L.M.K. is a consultant for Pfizer, Inc., Summit Corporation PLC, and Sarepta Therapeutics for muscle disease drug therapies.
Figures

References
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Monaco AP, et al. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323:646–650. - PubMed
-
- Emery AE, Muntoni F, Quinlivan RC. Duchenne Muscular Dystrophy. Oxford Univ Press; Oxford: 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

