Bidirectional regulation of Aβ levels by Presenilin 1
- PMID: 28533411
- PMCID: PMC5502639
- DOI: 10.1073/pnas.1705235114
Bidirectional regulation of Aβ levels by Presenilin 1
Abstract
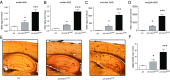
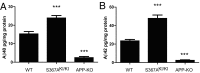
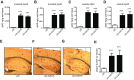
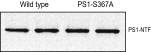
Alzheimer's disease (AD) is characterized by accumulation of the β-amyloid peptide (Aβ), which is generated through sequential proteolysis of the amyloid precursor protein (APP), first by the action of β-secretase, generating the β-C-terminal fragment (βCTF), and then by the Presenilin 1 (PS1) enzyme in the γ-secretase complex, generating Aβ. γ-Secretase is an intramembranous protein complex composed of Aph1, Pen2, Nicastrin, and Presenilin 1. Although it has a central role in the pathogenesis of AD, knowledge of the mechanisms that regulate PS1 function is limited. Here, we show that phosphorylation of PS1 at Ser367 does not affect γ-secretase activity, but has a dramatic effect on Aβ levels in vivo. We identified CK1γ2 as the endogenous kinase responsible for the phosphorylation of PS1 at Ser367. Inhibition of CK1γ leads to a decrease in PS1 Ser367 phosphorylation and an increase in Aβ levels in cultured cells. Transgenic mice in which Ser367 of PS1 was mutated to Ala, show dramatic increases in Aβ peptide and in βCTF levels in vivo. Finally, we show that this mutation impairs the autophagic degradation of βCTF, resulting in its accumulation and increased levels of Aβ peptide and plaque load in the brain. Our results demonstrate that PS1 regulates Aβ levels by a unique bifunctional mechanism. In addition to its known role as the catalytic subunit of the γ-secretase complex, selective phosphorylation of PS1 on Ser367 also decreases Aβ levels by increasing βCTF degradation through autophagy. Elucidation of the mechanism by which PS1 regulates βCTF degradation may aid in the development of potential therapies for Alzheimer's disease.
Keywords: Alzheimer’s disease; Aβ; Presenilin 1; autophagy; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Versatility of presenilin 1.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):6885-6887. doi: 10.1073/pnas.1707809114. Epub 2017 Jun 23. Proc Natl Acad Sci U S A. 2017. PMID: 28645897 Free PMC article. No abstract available.
Similar articles
-
Phosphorylated Presenilin 1 decreases β-amyloid by facilitating autophagosome-lysosome fusion.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):7148-7153. doi: 10.1073/pnas.1705240114. Epub 2017 May 22. Proc Natl Acad Sci U S A. 2017. PMID: 28533369 Free PMC article.
-
Identification of new Presenilin-1 phosphosites: implication for γ-secretase activity and Aβ production.J Neurochem. 2015 May;133(3):409-21. doi: 10.1111/jnc.12996. Epub 2015 Feb 24. J Neurochem. 2015. PMID: 25458374
-
Hydrophilic loop 1 of Presenilin-1 and the APP GxxxG transmembrane motif regulate γ-secretase function in generating Alzheimer-causing Aβ peptides.J Biol Chem. 2021 Jan-Jun;296:100393. doi: 10.1016/j.jbc.2021.100393. Epub 2021 Feb 8. J Biol Chem. 2021. PMID: 33571524 Free PMC article.
-
Amyloidogenic and anti-amyloidogenic properties of presenilin 1.Adv Pharmacol. 2021;90:239-251. doi: 10.1016/bs.apha.2020.09.010. Epub 2020 Oct 24. Adv Pharmacol. 2021. PMID: 33706935 Review.
-
Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review.
Cited by
-
Presenilins and γ-Secretase in Membrane Proteostasis.Cells. 2019 Mar 1;8(3):209. doi: 10.3390/cells8030209. Cells. 2019. PMID: 30823664 Free PMC article. Review.
-
Is γ-secretase a beneficial inactivating enzyme of the toxic APP C-terminal fragment C99?J Biol Chem. 2021 Jan-Jun;296:100489. doi: 10.1016/j.jbc.2021.100489. Epub 2021 Mar 1. J Biol Chem. 2021. PMID: 33662398 Free PMC article. Review.
-
Presenilin 1 phosphorylation regulates amyloid-β degradation by microglia.Mol Psychiatry. 2021 Oct;26(10):5620-5635. doi: 10.1038/s41380-020-0856-8. Epub 2020 Aug 13. Mol Psychiatry. 2021. PMID: 32792660 Free PMC article.
-
The Nuclear Lamina: Protein Accumulation and Disease.Biomedicines. 2020 Jul 1;8(7):188. doi: 10.3390/biomedicines8070188. Biomedicines. 2020. PMID: 32630170 Free PMC article. Review.
-
Dynamic changes of autophagic flux induced by Abeta in the brain of postmortem Alzheimer's disease patients, animal models and cell models.Aging (Albany NY). 2020 Jun 13;12(11):10912-10930. doi: 10.18632/aging.103305. Epub 2020 Jun 13. Aging (Albany NY). 2020. PMID: 32535554 Free PMC article.
References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Cai H, et al. BACE1 is the major β-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. - PubMed
-
- De Strooper B, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. - PubMed
-
- Prager K, et al. A structural switch of presenilin 1 by glycogen synthase kinase 3β-mediated phosphorylation regulates the interaction with β-catenin and its nuclear signaling. J Biol Chem. 2007;282:14083–14093. - PubMed
-
- Lau K-F, et al. Cyclin-dependent kinase-5/p35 phosphorylates Presenilin 1 to regulate carboxy-terminal fragment stability. Mol Cell Neurosci. 2002;20:13–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

