Manipulating DNA damage-response signaling for the treatment of immune-mediated diseases
- PMID: 28533414
- PMCID: PMC5474825
- DOI: 10.1073/pnas.1703683114
Manipulating DNA damage-response signaling for the treatment of immune-mediated diseases
Abstract
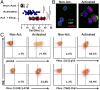
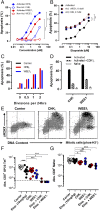
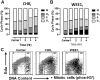
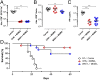
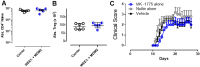
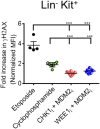
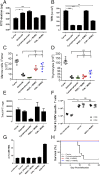
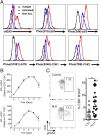
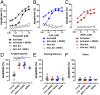
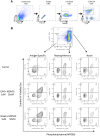
Antigen-activated lymphocytes undergo extraordinarily rapid cell division in the course of immune responses. We hypothesized that this unique aspect of lymphocyte biology leads to unusual genomic stress in recently antigen-activated lymphocytes and that targeted manipulation of DNA damage-response (DDR) signaling pathways would allow for selective therapeutic targeting of pathological T cells in disease contexts. Consistent with these hypotheses, we found that activated mouse and human T cells display a pronounced DDR in vitro and in vivo. Upon screening a variety of small-molecule compounds, we found that potentiation of p53 (via inhibition of MDM2) or impairment of cell cycle checkpoints (via inhibition of CHK1/2 or WEE1) led to the selective elimination of activated, pathological T cells in vivo. The combination of these strategies [which we termed "p53 potentiation with checkpoint abrogation" (PPCA)] displayed therapeutic benefits in preclinical disease models of hemophagocytic lymphohistiocytosis and multiple sclerosis, which are driven by foreign antigens or self-antigens, respectively. PPCA therapy targeted pathological T cells but did not compromise naive, regulatory, or quiescent memory T-cell pools, and had a modest nonimmune toxicity profile. Thus, PPCA is a therapeutic modality for selective, antigen-specific immune modulation with significant translational potential for diverse immune-mediated diseases.
Keywords: DNA damage response; autoimmunity; immune regulation; therapeutics.
Conflict of interest statement
Conflict of interest statement: M.B.J. is an inventor on a patent filing related to this work. The authors have no other conflicts to disclose.
Figures




References
-
- Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol. 2006;24:497–518. - PubMed
-
- Seto T, et al. Phase I, dose-escalation study of AZD7762 alone and in combination with gemcitabine in Japanese patients with advanced solid tumours. Cancer Chemother Pharmacol. 2013;72:619–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

