First-Line Palliative HER2-Targeted Therapy in HER2-Positive Metastatic Breast Cancer Is Less Effective After Previous Adjuvant Trastuzumab-Based Therapy
- PMID: 28533475
- PMCID: PMC5553959
- DOI: 10.1634/theoncologist.2016-0448
First-Line Palliative HER2-Targeted Therapy in HER2-Positive Metastatic Breast Cancer Is Less Effective After Previous Adjuvant Trastuzumab-Based Therapy
Abstract
Background: Survival of patients with human epidermal growth receptor 2 (HER2)-positive metastatic breast cancer (MBC) has improved dramatically since trastuzumab has become available, although the disease eventually progresses in most patients. This study investigates the outcome (overall survival [OS] and time to next treatment [TNT]) in MBC patients pretreated with trastuzumab in the adjuvant setting (TP-group) compared with trastuzumab-naïve patients (TN-group) in order to investigate the possibility of trastuzumab resistance.
Patients and methods: Patients treated with first-line HER2-targeted-containing chemotherapy were eligible for the study. A power analysis was performed to estimate the minimum size of the TP-group. OS and TNT were estimated using Kaplan-Meier curves and multivariable Cox proportional hazards models.
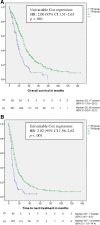
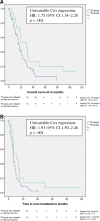
Results: Between January 1, 2000, and June 1, 2014, 469 patients were included, of whom 82 were in the TP-group and 387 were in the TN-group. Median OS and TNT were significantly worse in the TP-group compared with the TN-group (17 vs. 30 months, adjusted hazard ratio [HR] 1.84 [1.15-2.96], p = .01 and 7 vs. 13 months, adjusted HR 1.65 [1.06-2.58], p = .03) after adjustment for age, year of diagnosis, disease-free interval, hormone receptor status, metastatic site, and cytotoxic regimens.
Conclusion: First-line trastuzumab-containing treatment regimens are less effective in patients with failure of adjuvant trastuzumab compared with trastuzumab-naïve patients and might be due to trastuzumab resistance. The impact of trastuzumab resistance on the response on dual HER2 blockade with trastuzumab and pertuzumab and how resistance mechanisms can be used in the optimization of HER2-targeted treatment lines need further investigation.
Implications for practice: Evidence on the efficacy of palliative trastuzumab-based therapy after failure of trastuzumab in the adjuvant setting is limited because of a minority of patients treated with adjuvant trastuzumab in clinical trials. In this study, less clinical benefit of palliative trastuzumab-based therapy was observed in patients relapsing after adjuvant trastuzumab compared with no adjuvant trastuzumab treatment. Subgroup analyses and multivariable analyses revealed that this was independent of possible confounding factors, including adjuvant taxane-treatment. This might suggest a clinically meaningful impaired efficacy of trastuzumab after previous, in this case adjuvant, trastuzumab therapy. These results could have implications for treatment decision-making after short progression-free intervals on trastuzumab-containing regimens in the palliative setting.
摘要
背景. 自曲妥珠单抗问世后, 人表皮生长因子受体2(HER2)阳性转移性乳腺癌(MBC)患者的生存期大幅改善, 但大多数患者最终仍发生疾病进展。本研究比较了既往接受过曲妥珠单抗辅助治疗的MBC患者(TP组)与曲妥珠单抗初治患者(TN组)的预后[总生存期(OS)和至下次治疗时间(TNT)], 以考察曲妥珠单抗耐药的可能性。
患者和方法. 接受含HER2靶向药物一线化疗的患者有资格参与研究。通过把握度分析来估算TP组的最小样本量。使用Kaplan‐Meier曲线和多变量Cox比例风险模型估计OS和TNT。
结果. 在2000年1月1日至2014年6月1日期间入组了469例患者, 其中TP组82例, TN组387例。校正年龄、诊断年数、无病间期、激素受体状态、转移部位和细胞毒性药物治疗方案后, TP组的中位OS和TNT显著短于TN组[17个月 vs. 30个月, 校正风险比(HR)=1.84(1.15‐2.96), p=0.01;7个月 vs. 13个月, 校正HR=1.65(1.06–2.58), p=0.03]。
结论. 与曲妥珠单抗初治患者相比, 既往曲妥珠单抗辅助治疗失败的患者使用曲妥珠单抗一线治疗方案时疗效有所下降, 这可能是由于曲妥珠单抗耐药所致。需要进一步研究曲妥珠单抗耐药对曲妥珠单抗和帕妥珠单抗双重HER2阻滞疗效的影响以及如何利用耐药机制优化HER2靶向治疗方案。
Keywords: Human epidermal growth receptor 2 positive; Metastatic breast cancer; Overall survival; Trastuzumab.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Slamon DJ, Eiermann W, Robert NJ et al. Ten year follow‐up of the BCIRG‐006 trial comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC+T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC+TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer patients. SABCS 2015;S5‐04a.
-
- Eiermann W, International Herceptin Study Group. Trastuzumab combined with chemotherapy for the treatment of HER2‐positive metastatic breast cancer: Pivotal trial data. Ann Oncol 2001;12(suppl 1):S57–S62. - PubMed
-
- Murthy RK, Varma A, Mishra P et al. Effect of adjuvant/neoadjuvant trastuzumab on clinical outcomes in patients with HER2‐positive metastatic breast cancer. Cancer 2014;120:1932–1938. - PubMed
-
- Vaz‐Luis I, Seah D, Olson EM et al. Clinicopathological features among patients with advanced human epidermal growth factor‐2‐positive breast cancer with prolonged clinical benefit to first‐line trastuzumab‐based therapy: A retrospective cohort study. Clin Breast Cancer 2013;13:254–263. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

