Effect of epigallocatechin-3-gallate, major ingredient of green tea, on the pharmacokinetics of rosuvastatin in healthy volunteers
- PMID: 28533679
- PMCID: PMC5431696
- DOI: 10.2147/DDDT.S130050
Effect of epigallocatechin-3-gallate, major ingredient of green tea, on the pharmacokinetics of rosuvastatin in healthy volunteers
Abstract
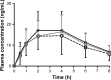
Previous in vitro studies have demonstrated the inhibitory effect of green tea on drug transporters. Because rosuvastatin, a lipid-lowering drug widely used for the prevention of cardiovascular events, is a substrate for many drug transporters, there is a possibility that there is interaction between green tea and rosuvastatin. The aim of this study was to investigate the effect of green tea on the pharmacokinetics of rosuvastatin in healthy volunteers. An open-label, three-treatment, fixed-sequence study was conducted. On Day 1, 20 mg of rosuvastatin was given to all subjects. After a 3-day washout period, the subjects received 20 mg of rosuvastatin plus 300 mg of epigallocatechin-3-gallate (EGCG), a major ingredient of green tea (Day 4). After a 10-day pretreatment of EGCG up to Day 14, they received rosuvastatin (20 mg) plus EGCG (300 mg) once again (Day 15). Blood samples for the pharmacokinetic assessments were collected up to 8 hours after each dose of rosuvastatin. A total of 13 healthy volunteers were enrolled. Compared with the administration of rosuvastatin alone, the concomitant use at Day 4 significantly reduced the area under the concentration-time curve from time 0 to the last measurable time (AUClast) by 19% (geometric mean ratio 0.81, 90% confidence interval [CI] 0.67-0.97) and the peak plasma concentration (Cmax) by 15% (geometric mean ratio 0.85, 90% CI 0.70-1.04). AUClast or Cmax of rosuvastatin on Day 15 was not significantly different from that on Day 1. This study demonstrated that co-administration of EGCG reduces the systemic exposure of rosuvastatin by 19%, and pretreatment of EGCG can eliminate that effect of co-administration of EGCG.
Keywords: EGCG; drug interaction; drug transporter; green tea; pharmacokinetics; rosuvastatin.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Effect of Green Tea and (-)-Epigallocatechin Gallate on the Pharmacokinetics of Rosuvastatin.Curr Drug Metab. 2020;21(6):471-478. doi: 10.2174/1389200221666200514133355. Curr Drug Metab. 2020. PMID: 32407265
-
Lack of pharmacokinetic interaction between fluvastatin and green tea in healthy volunteers.Eur J Clin Pharmacol. 2018 May;74(5):601-609. doi: 10.1007/s00228-018-2420-x. Epub 2018 Jan 24. Eur J Clin Pharmacol. 2018. PMID: 29368187 Clinical Trial.
-
Evidence for a pharmacokinetic interaction between eslicarbazepine and rosuvastatin: Potential effects on xenobiotic transporters.Epilepsy Res. 2017 Sep;135:64-70. doi: 10.1016/j.eplepsyres.2017.05.005. Epub 2017 May 18. Epilepsy Res. 2017. PMID: 28624574 Clinical Trial.
-
Inhibitory Effects of Eight Green Tea Catechins on Cytochrome P450 1A2, 2C9, 2D6, and 3A4 Activities.J Pharm Pharm Sci. 2016 Apr-Jun;19(2):188-97. doi: 10.18433/J3MS5C. J Pharm Pharm Sci. 2016. PMID: 27518169 Review.
-
Effects of green tea and EGCG on cardiovascular and metabolic health.J Am Coll Nutr. 2007 Aug;26(4):373S-388S. doi: 10.1080/07315724.2007.10719626. J Am Coll Nutr. 2007. PMID: 17906191 Review.
Cited by
-
Effect of Green Tea Extract and Soy Isoflavones on the Pharmacokinetics of Rosuvastatin in Healthy Volunteers.Front Nutr. 2022 Mar 24;9:850318. doi: 10.3389/fnut.2022.850318. eCollection 2022. Front Nutr. 2022. PMID: 35399656 Free PMC article.
-
Lipid-lowering statins and polyphenol-based supplementation: a scoping review on drug-food interaction potential.Front Pharmacol. 2025 May 30;16:1541871. doi: 10.3389/fphar.2025.1541871. eCollection 2025. Front Pharmacol. 2025. PMID: 40520191 Free PMC article.
-
The involvement of human organic anion transporting polypeptides (OATPs) in drug-herb/food interactions.Chin Med. 2020 Jul 9;15:71. doi: 10.1186/s13020-020-00351-9. eCollection 2020. Chin Med. 2020. PMID: 32670395 Free PMC article. Review.
-
HDI Highlighter, The First Intelligent Tool to Screen the Literature on Herb-Drug Interactions.Clin Pharmacokinet. 2022 Jun;61(6):761-788. doi: 10.1007/s40262-022-01131-4. Epub 2022 May 31. Clin Pharmacokinet. 2022. PMID: 35637377 Review.
-
Transporter-mediated natural product-drug interactions for the treatment of cardiovascular diseases.J Food Drug Anal. 2018 Apr;26(2S):S32-S44. doi: 10.1016/j.jfda.2017.11.008. Epub 2017 Dec 19. J Food Drug Anal. 2018. PMID: 29703385 Free PMC article. Review.
References
-
- Werba JP, Misaka S, Giroli MG, et al. Overview of green tea interaction with cardiovascular drugs. Curr Pharm Des. 2015;21(9):1213–1219. - PubMed
-
- Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17(7):573–586. - PubMed
-
- Kuriyama S, Shimazu T, Ohmori K, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296(10):1255–1265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

