Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges
- PMID: 28533743
- PMCID: PMC5420571
- DOI: 10.3389/fncel.2017.00078
Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges
Abstract
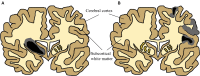
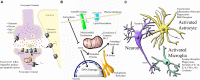
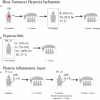
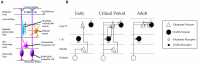
Neonatal hypoxia-ischaemia (HI) is the most common cause of death and disability in human neonates, and is often associated with persistent motor, sensory, and cognitive impairment. Improved intensive care technology has increased survival without preventing neurological disorder, increasing morbidity throughout the adult population. Early preventative or neuroprotective interventions have the potential to rescue brain development in neonates, yet only one therapeutic intervention is currently licensed for use in developed countries. Recent investigations of the transient cortical layer known as subplate, especially regarding subplate's secretory role, opens up a novel set of potential molecular modulators of neonatal HI injury. This review examines the biological mechanisms of human neonatal HI, discusses evidence for the relevance of subplate-secreted molecules to this condition, and evaluates available animal models. Neuroserpin, a neuronally released neuroprotective factor, is discussed as a case study for developing new potential pharmacological interventions for use post-ischaemic injury.
Keywords: encephalopathy; hypoxia-ischemia; neonatal; neurodevelopment; neuroprotection; neuroserpin; subplate.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

