Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL
- PMID: 28533941
- PMCID: PMC5437652
- DOI: 10.1186/s40164-017-0074-5
Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL
Abstract
Background: Blinatumomab has shown a remission rate of 69% in an exploratory single-arm, phase II dose-escalation study in adult patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL). We evaluated changes in laboratory parameters and immunopharmacodynamic markers in patients who received blinatumomab in the exploratory phase II study.
Methods: Data from 36 adults with relapsed/refractory ALL receiving blinatumomab as 4-week continuous IV infusions in various dose cohorts were analyzed for changes in liver enzymes, first-dose parameters, peripheral blood cell subpopulations, and cytokine/granzyme B release. Associations with clinical response were evaluated.
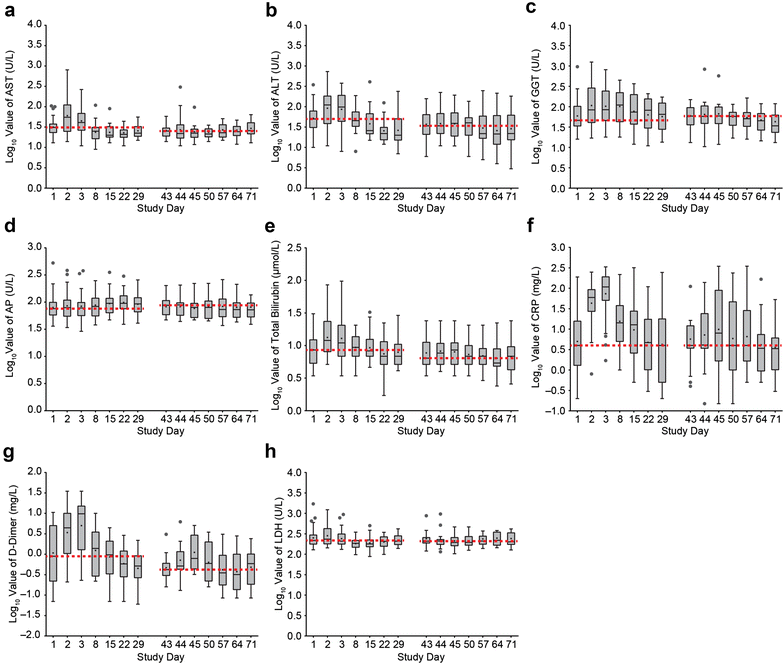
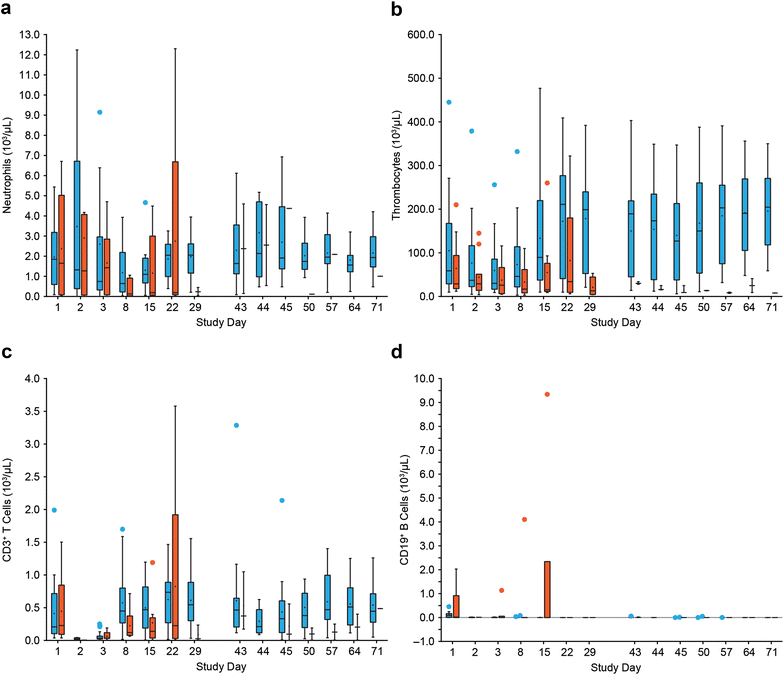
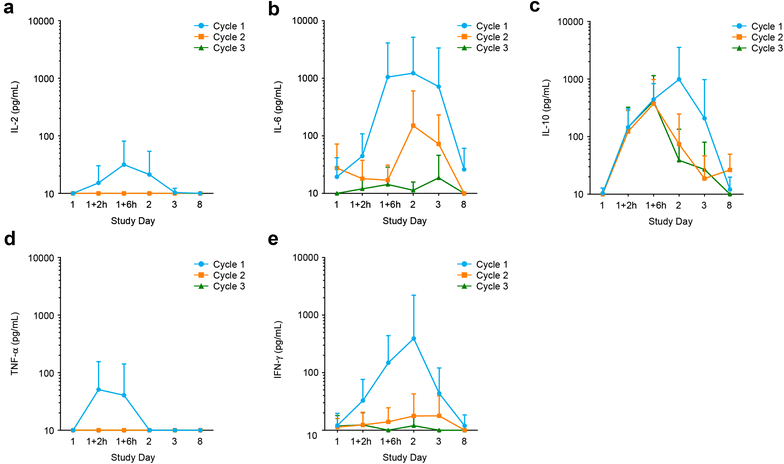
Results: Liver enzymes and inflammatory parameters transiently increased primarily during the first treatment week without clinical symptoms and reversed to baseline levels thereafter. B and T cells showed expected depletion and redistribution kinetics, respectively. Similarly, thrombocytes and T cells displayed an initial decline in cell counts, whereas neutrophils peaked during the first days after infusion start. T-cell redistribution coincided with upregulation of LFA-1 and CD69. Patients who responded to blinatumomab had more pronounced T-cell expansion, which was associated with proliferation of CD4+ and CD8+ T cells and memory subsets. Release of cytokines and granzyme B primarily occurred during the first week of cycle 1, except for IL-10, which was released in subsequent cycles. Blinatumomab step-dosing was associated with lower cytokine release and lower body temperature.
Conclusions: In this study of relapsed/refractory ALL, blinatumomab-induced changes in laboratory parameters were transient and reversible. The evaluated PD markers demonstrated blinatumomab activity, and the analysis of cytokines supported the rationale for stepwise dosing. (ClinicalTrials.gov Identifier NCT01209286.).
Keywords: Acute lymphoblastic leukemia; BiTE®; Bispecific; Blinatumomab; CD19; Liver enzymes; Pharmacodynamics.
Figures




References
-
- Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. - DOI - PubMed
-
- Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, Neumann SA, Horst HA, Raff T, Viardot A, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120:5185–5187. doi: 10.1182/blood-2012-07-441030. - DOI - PubMed
-
- Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, Noppeney R, Hess G, Kallert S, Mackensen A, et al. Bispecific T-Cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-hodgkin lymphoma: final results from a phase I Study. J Clin Oncol. 2016;34:1104–1111. doi: 10.1200/JCO.2014.59.1586. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

