White matter injury in the preterm infant: pathology and mechanisms
- PMID: 28534077
- PMCID: PMC5973818
- DOI: 10.1007/s00401-017-1718-6
White matter injury in the preterm infant: pathology and mechanisms
Abstract
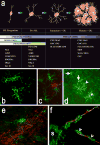
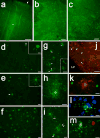
The human preterm brain is particularly susceptible to cerebral white matter injury (WMI) that disrupts the normal progression of developmental myelination. Advances in the care of preterm infants have resulted in a sustained reduction in the severity of WMI that has shifted from more severe focal necrotic lesions to milder diffuse WMI. Nevertheless, WMI remains a global health problem and the most common cause of chronic neurological morbidity from cerebral palsy and diverse neurobehavioral disabilities. Diffuse WMI involves maturation-dependent vulnerability of the oligodendrocyte (OL) lineage with selective degeneration of late oligodendrocyte progenitors (preOLs) triggered by oxidative stress and other insults. The magnitude and distribution of diffuse WMI are related to both the timing of appearance and regional distribution of susceptible preOLs. Diffuse WMI disrupts the normal progression of OL lineage maturation and myelination through aberrant mechanisms of regeneration and repair. PreOL degeneration is accompanied by early robust proliferation of OL progenitors that regenerate and augment the preOL pool available to generate myelinating OLs. However, newly generated preOLs fail to differentiate and initiate myelination along their normal developmental trajectory despite the presence of numerous intact-appearing axons. Disrupted preOL maturation is accompanied by diffuse gliosis and disturbances in the composition of the extracellular matrix and is mediated in part by inhibitory factors derived from reactive astrocytes. Signaling pathways implicated in disrupted myelination include those mediated by Notch, WNT-beta catenin, and hyaluronan. Hence, there exists a potentially broad but still poorly defined developmental window for interventions to promote white matter repair and myelination and potentially reverses the widespread disturbances in cerebral gray matter growth that accompanies WMI.
Keywords: Astrocyte; Development; Dysmaturation; Glia; Hypoxia–ischemia; MRI; Microglia; Oligodendrocyte; Periventricular leukomalacia; Prematurity.
Figures


References
-
- (CDC) CfDCaP. Economic costs associated with mental retardation, cerebral palsy, hearing loss and vision impairment–United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:57–59. - PubMed
-
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. - PubMed
-
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. - PubMed
-
- Alix JJ, Fern R. Glutamate receptor-mediated ischemic injury of premyelinated central axons. Ann Neurol. 2009;66:682–693. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

