Splenic Ly6Chi monocytes contribute to adverse late post-ischemic left ventricular remodeling in heme oxygenase-1 deficient mice
- PMID: 28534119
- PMCID: PMC5440541
- DOI: 10.1007/s00395-017-0629-y
Splenic Ly6Chi monocytes contribute to adverse late post-ischemic left ventricular remodeling in heme oxygenase-1 deficient mice
Abstract
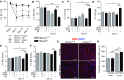
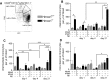
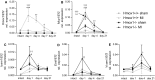
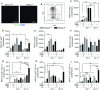
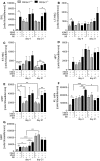
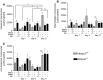
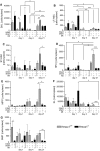
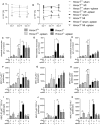
Heme oxygenase-1 (Hmox1) is a stress-inducible protein crucial in heme catabolism. The end products of its enzymatic activity possess anti-oxidative, anti-apoptotic and anti-inflammatory properties. Cardioprotective effects of Hmox1 were demonstrated in experimental models of myocardial infarction (MI). Nevertheless, its importance in timely resolution of post-ischemic inflammation remains incompletely understood. The aim of this study was to determine the role of Hmox1 in the monocyte/macrophage-mediated cardiac remodeling in a mouse model of MI. Hmox1 knockout (Hmox1-/-) and wild-type (WT, Hmox1+/+) mice were subjected to a permanent ligation of the left anterior descending coronary artery. Significantly lower incidence of left ventricle (LV) free wall rupture was noted between 3rd and 5th day after MI in Hmox1-/- mice resulting in their better overall survival. Then, starting from 7th until 21st day post-MI a more potent deterioration of LV function was observed in Hmox1-/- than in the surviving Hmox1+/+ mice. This was accompanied by higher numbers of Ly6Chi monocytes in peripheral blood, as well as higher expression of monocyte chemoattractant protein-1 and adhesion molecules in the hearts of MI-operated Hmox1-/- mice. Consequently, a greater post-MI monocyte-derived myocardial macrophage infiltration was noted in Hmox1-deficient individuals. Splenectomy decreased the numbers of circulating inflammatory Ly6Chi monocytes in blood, reduced the numbers of proinflammatory cardiac macrophages and significantly improved the post-MI LV function in Hmox1-/- mice. In conclusion, Hmox1 deficiency has divergent consequences in MI. On the one hand, it improves early post-MI survival by decreasing the occurrence of cardiac rupture. Afterwards, however, the hearts of Hmox1-deficient mice undergo adverse late LV remodeling due to overactive and prolonged post-ischemic inflammatory response. We identified spleen as an important source of these cardiovascular complications in Hmox1-/- mice.
Keywords: Cardiac rupture; Heme oxygenase-1; Macrophages; Monocytes; Myocardial infarction.
Conflict of interest statement
Funding
This work was supported by Grants: Homing-Plus/2011-3/3 from the Foundation for Polish Science, 0249/IP1/2013/72 from the Ministry of Science and Higher Education, 2014/14/E/NZ1/00139 from the Polish National Science Center (to A. Jazwa) and 2015/17/N/NZ1/00041 from the Polish National Science Center (to M. Tomczyk). Faculty of Biochemistry, Biophysics and Biotechnology of Jagiellonian University is a partner of the Leading National Research Center (KNOW) supported by the Ministry of Science and Higher Education.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures

Similar articles
-
Calpastatin overexpression impairs postinfarct scar healing in mice by compromising reparative immune cell recruitment and activation.Am J Physiol Heart Circ Physiol. 2015 Dec 1;309(11):H1883-93. doi: 10.1152/ajpheart.00594.2015. Epub 2015 Oct 9. Am J Physiol Heart Circ Physiol. 2015. PMID: 26453333
-
Topiramate modulates post-infarction inflammation primarily by targeting monocytes or macrophages.Cardiovasc Res. 2017 Apr 1;113(5):475-487. doi: 10.1093/cvr/cvx027. Cardiovasc Res. 2017. PMID: 28339742
-
The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction.Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2206-13. doi: 10.1161/ATVBAHA.112.254409. Epub 2012 Jul 12. Arterioscler Thromb Vasc Biol. 2012. PMID: 22796582
-
Modulation of the monocyte/macrophage system in heart failure by targeting heme oxygenase-1.Vascul Pharmacol. 2019 Jan;112:79-90. doi: 10.1016/j.vph.2018.08.011. Epub 2018 Sep 10. Vascul Pharmacol. 2019. PMID: 30213580 Review.
-
Post-infarction inflammation and left ventricular remodeling: a double-edged sword.Circ J. 2013;77(3):580-7. doi: 10.1253/circj.cj-13-0013. Epub 2013 Jan 29. Circ J. 2013. PMID: 23358460 Review.
Cited by
-
Intravital imaging of splenic classical monocytes modifying the hepatic CX3CR1+ cells motility to exacerbate liver fibrosis via spleen-liver axis.Theranostics. 2024 Mar 3;14(5):2210-2231. doi: 10.7150/thno.87791. eCollection 2024. Theranostics. 2024. PMID: 38505603 Free PMC article.
-
Role of Heme-Oxygenase-1 in Biology of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells.Cells. 2021 Mar 1;10(3):522. doi: 10.3390/cells10030522. Cells. 2021. PMID: 33804563 Free PMC article.
-
Immunometabolism in heart failure.Nat Rev Cardiol. 2025 Jun 22. doi: 10.1038/s41569-025-01165-8. Online ahead of print. Nat Rev Cardiol. 2025. PMID: 40544171 Review.
-
Inflammatory cells and their non-coding RNAs as targets for treating myocardial infarction.Basic Res Cardiol. 2018 Dec 6;114(1):4. doi: 10.1007/s00395-018-0712-z. Basic Res Cardiol. 2018. PMID: 30523422 Free PMC article. Review.
-
Mapping macrophage polarization over the myocardial infarction time continuum.Basic Res Cardiol. 2018 Jun 4;113(4):26. doi: 10.1007/s00395-018-0686-x. Basic Res Cardiol. 2018. PMID: 29868933 Free PMC article.
References
-
- Ahn D, Cheng L, Moon C, Spurgeon H, Lakatta EG, Talan MI. Induction of myocardial infarcts of a predictable size and location by branch pattern probability-assisted coronary ligation in C57BL/6 mice. Am J Physiol Heart Circ Physiol. 2004;286:H1201–H1207. doi: 10.1152/ajpheart.00862.2003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

