Protective Effects of Neural Crest-Derived Stem Cell-Conditioned Media against Ischemia-Reperfusion-Induced Lung Injury in Rats
- PMID: 28534140
- PMCID: PMC7102066
- DOI: 10.1007/s10753-017-0594-5
Protective Effects of Neural Crest-Derived Stem Cell-Conditioned Media against Ischemia-Reperfusion-Induced Lung Injury in Rats
Abstract
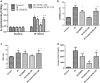
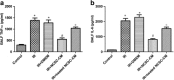
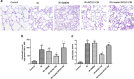
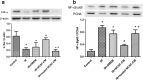
Current treatments for ischemia-reperfusion (IR)-induced acute lung injury are limited. Mesenchymal stem cell-conditioned medium (CM) has been reported to attenuate lung injury. Neural crest stem cells (NCSCs), a type of multipotent stem cells, are more easily obtained than mesenchymal stem cells. We hypothesize that NCSC-CM has anti-inflammatory properties that could protect against IR-induced lung injury in rats. In this study, NCSC-CM was derived from rat NCSCs. Typical acute lung injury was induced by 30-min ischemia followed by 90-min reperfusion in adult male Sprague-Dawley rats. Bronchoalveolar lavage fluid (BALF) and lung tissues were collected to analyze the degree of lung injury after the experiment. NCSC-CM was administered before ischemia and after reperfusion. NCSC-CM treatment significantly attenuated IR-induced lung edema, as indicated by decreases in pulmonary vascular permeability, lung weight gain, wet to dry weight ratio, lung weight to body weight ratio, pulmonary arterial pressure, and protein level in BALF. The levels of tumor necrosis factor-α and interleukin-6 in the BALF were also significantly decreased. Additionally, NCSC-CM improved lung pathology and neutrophil infiltration in the lung tissue, and significantly suppressed nuclear factor (NF)-κB activity and IκB-α degradation in the lung. However, heating NCSC-CM eliminated these protective effects. Our experiment demonstrates that NCSC-CM treatment decreases IR-induced acute lung injury and that the protective mechanism may be attributable to the inhibition of NF-κB activation and the inflammatory response. Therefore, NCSC-CM may be a novel approach for treating IR-induced lung injury.
Keywords: acute lung injury; conditioned media; ischemia-reperfusion; neural crest stem cell.
Figures



Similar articles
-
Protective effect of hypercapnic acidosis in ischemia-reperfusion lung injury is attributable to upregulation of heme oxygenase-1.PLoS One. 2013 Sep 10;8(9):e74742. doi: 10.1371/journal.pone.0074742. eCollection 2013. PLoS One. 2013. PMID: 24040332 Free PMC article.
-
Glutamine protects ischemia-reperfusion induced acute lung injury in isolated rat lungs.Pulm Pharmacol Ther. 2011 Feb;24(1):153-61. doi: 10.1016/j.pupt.2010.07.002. Epub 2010 Aug 3. Pulm Pharmacol Ther. 2011. PMID: 20688185
-
Protection against reperfusion lung injury via aborgating multiple signaling cascades by trichostatin A.Int Immunopharmacol. 2015 Apr;25(2):267-75. doi: 10.1016/j.intimp.2015.02.013. Epub 2015 Feb 16. Int Immunopharmacol. 2015. PMID: 25698558
-
Epidermal neural crest stem cells and their use in mouse models of spinal cord injury.Brain Res Bull. 2010 Oct 30;83(5):189-93. doi: 10.1016/j.brainresbull.2010.07.002. Epub 2010 Jul 14. Brain Res Bull. 2010. PMID: 20637266 Review.
-
Human epidermal neural crest stem cells as candidates for cell-based therapies, disease modeling, and drug discovery.Birth Defects Res C Embryo Today. 2014 Sep;102(3):221-6. doi: 10.1002/bdrc.21073. Epub 2014 Sep 16. Birth Defects Res C Embryo Today. 2014. PMID: 25228472 Review.
Cited by
-
The Role of Extracellular Vesicles in Cutaneous Remodeling and Hair Follicle Dynamics.Int J Mol Sci. 2019 Jun 5;20(11):2758. doi: 10.3390/ijms20112758. Int J Mol Sci. 2019. PMID: 31195626 Free PMC article. Review.
-
The efficacy of mesenchymal stromal cell-derived therapies for acute respiratory distress syndrome-a meta-analysis of preclinical trials.Respir Res. 2020 Nov 20;21(1):307. doi: 10.1186/s12931-020-01574-y. Respir Res. 2020. PMID: 33218340 Free PMC article.
-
Effect of Placenta-Derived Mesenchymal Stromal Cells Conditioned Media on an LPS-Induced Mouse Model of Preeclampsia.Int J Mol Sci. 2022 Jan 31;23(3):1674. doi: 10.3390/ijms23031674. Int J Mol Sci. 2022. PMID: 35163594 Free PMC article.
-
Bone marrow mesenchymal stem cell-conditioned medium facilitates fluid resolution via miR-214-activating epithelial sodium channels.MedComm (2020). 2020 Nov 12;1(3):376-385. doi: 10.1002/mco2.40. eCollection 2020 Dec. MedComm (2020). 2020. PMID: 34766129 Free PMC article.
-
Gens PSD-95 and GSK-3β expression improved by hair follicular stem cells-conditioned medium enhances synaptic transmission and cognitive abilities in the rat model of vascular dementia.Brain Behav. 2024 Jan;14(1):e3351. doi: 10.1002/brb3.3351. Brain Behav. 2024. PMID: 38376050 Free PMC article.
References
-
- Curley GF, Ansari B, Hayes M, Devaney J, Masterson C, Ryan A, Barry F, O'Brien T, Toole DO, Laffey JG. Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology. 2013;118:924–932. doi: 10.1097/ALN.0b013e318287ba08. - DOI - PubMed
-
- Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. American Journal of Respiratory and Critical Care Medicine. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

