Intracellular and Extracellular Recording of Spontaneous Action Potentials in Mammalian Neurons and Cardiac Cells with 3D Plasmonic Nanoelectrodes
- PMID: 28534411
- PMCID: PMC5520104
- DOI: 10.1021/acs.nanolett.7b01523
Intracellular and Extracellular Recording of Spontaneous Action Potentials in Mammalian Neurons and Cardiac Cells with 3D Plasmonic Nanoelectrodes
Abstract
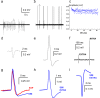
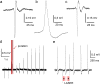
Three-dimensional vertical micro- and nanostructures can enhance the signal quality of multielectrode arrays and promise to become the prime methodology for the investigation of large networks of electrogenic cells. So far, access to the intracellular environment has been obtained via spontaneous poration, electroporation, or by surface functionalization of the micro/nanostructures; however, these methods still suffer from some limitations due to their intrinsic characteristics that limit their widespread use. Here, we demonstrate the ability to continuously record both extracellular and intracellular-like action potentials at each electrode site in spontaneously active mammalian neurons and HL-1 cardiac-derived cells via the combination of vertical nanoelectrodes with plasmonic optoporation. We demonstrate long-term and stable recordings with a very good signal-to-noise ratio. Additionally, plasmonic optoporation does not perturb the spontaneous electrical activity; it permits continuous recording even during the poration process and can regulate extracellular and intracellular contributions by means of partial cellular poration.
Keywords: Intracellular recording; cardiomyocytes; multielectrode arrays; neurons; plasmonic optoporation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

