The Soft- and Hard-Heartedness of Cardiac Fibroblasts: Mechanotransduction Signaling Pathways in Fibrosis of the Heart
- PMID: 28534817
- PMCID: PMC5447944
- DOI: 10.3390/jcm6050053
The Soft- and Hard-Heartedness of Cardiac Fibroblasts: Mechanotransduction Signaling Pathways in Fibrosis of the Heart
Abstract
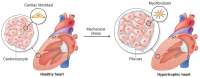
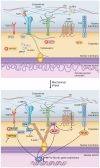
Cardiac fibrosis, the excessive accumulation of extracellular matrix (ECM), remains an unresolved problem in most forms of heart disease. In order to be successful in preventing, attenuating or reversing cardiac fibrosis, it is essential to understand the processes leading to ECM production and accumulation. Cardiac fibroblasts are the main producers of cardiac ECM, and harbor great phenotypic plasticity. They are activated by the disease-associated changes in mechanical properties of the heart, including stretch and increased tissue stiffness. Despite much remaining unknown, an interesting body of evidence exists on how mechanical forces are translated into transcriptional responses important for determination of fibroblast phenotype and production of ECM constituents. Such mechanotransduction can occur at multiple cellular locations including the plasma membrane, cytoskeleton and nucleus. Moreover, the ECM functions as a reservoir of pro-fibrotic signaling molecules that can be released upon mechanical stress. We here review the current status of knowledge of mechanotransduction signaling pathways in cardiac fibroblasts that culminate in pro-fibrotic gene expression.
Keywords: cardiac fibroblast; cytoskeleton; extracellular matrix; fibrosis; integrins; linker of the nucleoskeleton and cytoskeleton; mechanotransduction; myofibroblast; stiffness; syndecan.
Conflict of interest statement
ADM is a co-founder of and has an equity interest in Insilicomed, Inc. He serves as a scientific advisor to the company. Some research grants to ADM, including some of those acknowledged here, have been identified for conflict of interest management based on the overall scope of the project and its potential benefit to Insilicomed, Inc. ADM is required to disclose this relationship in publications acknowledging the grant support, however the research subject matter and findings reported do not involve the company in any way and they have no known relationship to the business activities or interests of the company. The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict of interest policies. The other authors have no competing interests to declare.
Figures

References
-
- Weber G.F., Zawaideh S., Hikita S., Kumar V.A., Cantor H., Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J. Leukoc. Biol. 2002;72:752–761. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

