Low- and High-LET Ionizing Radiation Induces Delayed Homologous Recombination that Persists for Two Weeks before Resolving
- PMID: 28535128
- PMCID: PMC5553630
- DOI: 10.1667/RR14748.1
Low- and High-LET Ionizing Radiation Induces Delayed Homologous Recombination that Persists for Two Weeks before Resolving
Abstract
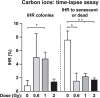
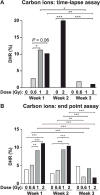
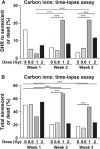
Genome instability is a hallmark of cancer cells and dysregulation or defects in DNA repair pathways cause genome instability and are linked to inherited cancer predisposition syndromes. Ionizing radiation can cause immediate effects such as mutation or cell death, observed within hours or a few days after irradiation. Ionizing radiation also induces delayed effects many cell generations after irradiation. Delayed effects include hypermutation, hyper-homologous recombination, chromosome instability and reduced clonogenic survival (delayed death). Delayed hyperrecombination (DHR) is mechanistically distinct from delayed chromosomal instability and delayed death. Using a green fluorescent protein (GFP) direct repeat homologous recombination system, time-lapse microscopy and colony-based assays, we demonstrate that DHR increases several-fold in response to low-LET X rays and high-LET carbon-ion radiation. Time-lapse analyses of DHR revealed two classes of recombinants not detected in colony-based assays, including cells that recombined and then senesced or died. With both low- and high-LET radiation, DHR was evident during the first two weeks postirradiation, but resolved to background levels during the third week. The results indicate that the risk of radiation-induced genome destabilization via DHR is time limited, and suggest that there is little or no additional risk of radiation-induced genome instability mediated by DHR with high-LET radiation compared to low-LET radiation.
Figures



Similar articles
-
UV radiation induces delayed hyperrecombination associated with hypermutation in human cells.Mol Cell Biol. 2006 Aug;26(16):6047-55. doi: 10.1128/MCB.00444-06. Mol Cell Biol. 2006. PMID: 16880516 Free PMC article.
-
Relative biological effectiveness of high linear energy transfer α-particles for the induction of DNA-double-strand breaks, chromosome aberrations and reproductive cell death in SW-1573 lung tumour cells.Oncol Rep. 2012 Mar;27(3):769-74. doi: 10.3892/or.2011.1604. Epub 2011 Dec 21. Oncol Rep. 2012. PMID: 22200791
-
Regulation of ATM in DNA double strand break repair accounts for the radiosensitivity in human cells exposed to high linear energy transfer ionizing radiation.Mutat Res. 2009 Nov 2;670(1-2):15-23. doi: 10.1016/j.mrfmmm.2009.06.016. Epub 2009 Jul 5. Mutat Res. 2009. PMID: 19583974
-
Genomic instability and the role of radiation quality.Radiat Prot Dosimetry. 2006;122(1-4):221-7. doi: 10.1093/rpd/ncl445. Epub 2006 Dec 12. Radiat Prot Dosimetry. 2006. PMID: 17164271 Review.
-
Radiation-induced genomic instability: radiation quality and dose response.Health Phys. 2003 Jul;85(1):23-9. doi: 10.1097/00004032-200307000-00006. Health Phys. 2003. PMID: 12852467 Review.
Cited by
-
Defective homologous recombination and genomic instability predict increased responsiveness to carbon ion radiotherapy in pancreatic cancer.NPJ Precis Oncol. 2025 Jan 17;9(1):20. doi: 10.1038/s41698-025-00800-4. NPJ Precis Oncol. 2025. PMID: 39824957 Free PMC article.
-
Reduction of Delayed Homologous Recombination by Induction of Radioadaptive Response in RaDR-GFP Mice (Yonezawa Effect): An Old Player With a New Role.Dose Response. 2019 Mar 4;17(1):1559325819833840. doi: 10.1177/1559325819833840. eCollection 2019 Jan-Mar. Dose Response. 2019. PMID: 30858771 Free PMC article.
-
Space Radiation Biology for "Living in Space".Biomed Res Int. 2020 Apr 8;2020:4703286. doi: 10.1155/2020/4703286. eCollection 2020. Biomed Res Int. 2020. PMID: 32337251 Free PMC article. Review.
-
Cosmic Ionizing Radiation: A DNA Damaging Agent That May Underly Excess Cancer in Flight Crews.Int J Mol Sci. 2024 Jul 12;25(14):7670. doi: 10.3390/ijms25147670. Int J Mol Sci. 2024. PMID: 39062911 Free PMC article. Review.
-
Replication stress and FOXM1 drive radiation induced genomic instability and cell transformation.PLoS One. 2020 Nov 30;15(11):e0235998. doi: 10.1371/journal.pone.0235998. eCollection 2020. PLoS One. 2020. PMID: 33253193 Free PMC article.
References
-
- Hawley L. Management of acute toxicity associated with radiotherapy. Br J Hosp Med (Lond) 2013;74:C173–6. - PubMed
-
- Hall EJ, Giaccia AJ. Radiobiology for the radiobiologist. 7th. Philadelphia: J. B. Lippincott; 2011.
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Allen CP, Fujimori A, Okayasu R, Nickoloff JA. Radiation-induced delayed genome instability and hypermutation in mammalian cells. In: Mittelman D, editor. Stress-induced mutagenesis. New York Heidelberg Dordrecht London: Springer; 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

