Characterization of liver injury, oval cell proliferation and cholangiocarcinogenesis in glutathione S-transferase A3 knockout mice
- PMID: 28535182
- PMCID: PMC5862260
- DOI: 10.1093/carcin/bgx048
Characterization of liver injury, oval cell proliferation and cholangiocarcinogenesis in glutathione S-transferase A3 knockout mice
Abstract
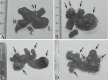
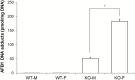
We recently generated glutathione S-transferase (GST) A3 knockout (KO) mice as a novel model to study the risk factors for liver cancer. GSTA3 KO mice are sensitive to the acute cytotoxic and genotoxic effects of aflatoxin B1 (AFB1), confirming the crucial role of GSTA3 in resistance to AFB1. We now report histopathological changes, tumor formation, biochemical changes and gender response following AFB1 treatment as well as the contribution of oxidative stress. Using a protocol of weekly 0.5 mg AFB1/kg administration, we observed extensive oval (liver stem) cell (OC) proliferation within 1-3 weeks followed by microvesicular lipidosis, megahepatocytes, nuclear inclusions, cholangiomas and small nodules. Male and female GSTA3 KO mice treated with 12 and 24 weekly AFB1 injections followed by a rest period of 12 and 6 months, respectively, all had grossly distorted livers with macro- and microscopic cysts, hepatocellular nodules, cholangiomas and cholangiocarcinomas and OC proliferation. We postulate that the prolonged AFB1 treatment leads to inhibition of hepatocyte proliferation, which is compensated by OC proliferation and eventually formation of cholangiocarcinoma (CCA). At low-dose AFB1, male KO mice showed less extensive acute liver injury, OC proliferation and AFB1-DNA adducts than female KO mice. There were no significant compensatory changes in KO mice GST subunits, GST enzymatic activity, epoxide hydrolase, or CYP1A2 and CYP3A11 levels. Finally, there was a modest increase in F2-isoprostane and isofuran in KO mice that confirmed putative GSTA3 hydroperoxidase activity in vivo for the first time.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





References
-
- Shupe T., et al. (2004) Low hepatic glutathione S-transferase and increased hepatic DNA adduction contribute to increased tumorigenicity of aflatoxin B1 in newborn and partially hepatectomized mice. Toxicol. Lett., 148, 1–9. - PubMed
-
- Eaton D.L., et al. (1994) Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol., 34, 135–172. - PubMed
-
- Egner P.A., et al. (2006) Quantification of aflatoxin-B1-N7-Guanine in human urine by high-performance liquid chromatography and isotope dilution tandem mass spectrometry. Chem. Res. Toxicol., 19, 1191–1195. - PubMed
-
- Hayes J.D., et al. (1994) Cloning of cDNAs from fetal rat liver encoding glutathione S-transferase Yc polypeptides. The Yc2 subunit is expressed in adult rat liver resistant to the hepatocarcinogen aflatoxin B1. J. Biol. Chem., 269, 20707–20717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

