Mms1 binds to G-rich regions in Saccharomyces cerevisiae and influences replication and genome stability
- PMID: 28535251
- PMCID: PMC5570088
- DOI: 10.1093/nar/gkx467
Mms1 binds to G-rich regions in Saccharomyces cerevisiae and influences replication and genome stability
Abstract
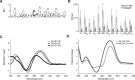
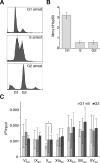
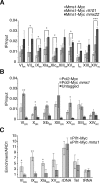
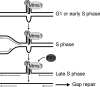
The regulation of replication is essential to preserve genome integrity. Mms1 is part of the E3 ubiquitin ligase complex that is linked to replication fork progression. By identifying Mms1 binding sites genome-wide in Saccharomyces cerevisiae we connected Mms1 function to genome integrity and replication fork progression at particular G-rich motifs. This motif can form G-quadruplex (G4) structures in vitro. G4 are stable DNA structures that are known to impede replication fork progression. In the absence of Mms1, genome stability is at risk at these G-rich/G4 regions as demonstrated by gross chromosomal rearrangement assays. Mms1 binds throughout the cell cycle to these G-rich/G4 regions and supports the binding of Pif1 DNA helicase. Based on these data we propose a mechanistic model in which Mms1 binds to specific G-rich/G4 motif located on the lagging strand template for DNA replication and supports Pif1 function, DNA replication and genome integrity.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

Similar articles
-
Mms1 is an assistant for regulating G-quadruplex DNA structures.Curr Genet. 2018 Jun;64(3):535-540. doi: 10.1007/s00294-017-0773-9. Epub 2017 Nov 2. Curr Genet. 2018. PMID: 29098365 Free PMC article. Review.
-
Pif1 is essential for efficient replisome progression through lagging strand G-quadruplex DNA secondary structures.Nucleic Acids Res. 2018 Dec 14;46(22):11847-11857. doi: 10.1093/nar/gky1065. Nucleic Acids Res. 2018. PMID: 30395308 Free PMC article.
-
DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase.Cell. 2011 May 27;145(5):678-91. doi: 10.1016/j.cell.2011.04.015. Cell. 2011. PMID: 21620135 Free PMC article.
-
Complementary roles of Pif1 helicase and single stranded DNA binding proteins in stimulating DNA replication through G-quadruplexes.Nucleic Acids Res. 2019 Sep 19;47(16):8595-8605. doi: 10.1093/nar/gkz608. Nucleic Acids Res. 2019. PMID: 31340040 Free PMC article.
-
Yeast Genome Maintenance by the Multifunctional PIF1 DNA Helicase Family.Genes (Basel). 2020 Feb 20;11(2):224. doi: 10.3390/genes11020224. Genes (Basel). 2020. PMID: 32093266 Free PMC article. Review.
Cited by
-
A Novel G-Quadruplex Binding Protein in Yeast-Slx9.Molecules. 2019 May 7;24(9):1774. doi: 10.3390/molecules24091774. Molecules. 2019. PMID: 31067825 Free PMC article.
-
RPA and Pif1 cooperate to remove G-rich structures at both leading and lagging strand.Cell Stress. 2020 Jan 17;4(3):48-63. doi: 10.15698/cst2020.03.214. Cell Stress. 2020. PMID: 32190820 Free PMC article.
-
Zuo1 supports G4 structure formation and directs repair toward nucleotide excision repair.Nat Commun. 2020 Aug 6;11(1):3907. doi: 10.1038/s41467-020-17701-8. Nat Commun. 2020. PMID: 32764578 Free PMC article.
-
Role of folding kinetics of secondary structures in telomeric G-overhangs in the regulation of telomere maintenance in Saccharomyces cerevisiae.J Biol Chem. 2020 Jul 3;295(27):8958-8971. doi: 10.1074/jbc.RA120.012914. Epub 2020 May 8. J Biol Chem. 2020. PMID: 32385108 Free PMC article.
-
Structure and function of Pif1 helicase.Biochem Soc Trans. 2017 Oct 15;45(5):1159-1171. doi: 10.1042/BST20170096. Epub 2017 Sep 12. Biochem Soc Trans. 2017. PMID: 28900015 Free PMC article. Review.
References
-
- Barbour L., Xiao W.. Regulation of alternative replication bypass pathways at stalled replication forks and its effects on genome stability: a yeast model. Mutat. Res. 2003; 532:137–155. - PubMed
-
- Luke B., Versini G., Jaquenoud M., Zaidi I.W., Kurz T., Pintard L., Pasero P., Peter M.. The cullin Rtt101p promotes replication fork progression through damaged DNA and natural pause sites. Curr. Biol.: CB. 2006; 16:786–792. - PubMed
-
- Duro E., Vaisica J.A., Brown G.W., Rouse J.. Budding yeast Mms22 and Mms1 regulate homologous recombination induced by replisome blockage. DNA Repair. 2008; 7:811–818. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

