Altered organization of the intermediate filament cytoskeleton and relocalization of proteostasis modulators in cells lacking the ataxia protein sacsin
- PMID: 28535259
- PMCID: PMC5886247
- DOI: 10.1093/hmg/ddx197
Altered organization of the intermediate filament cytoskeleton and relocalization of proteostasis modulators in cells lacking the ataxia protein sacsin
Abstract
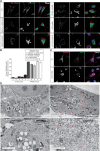
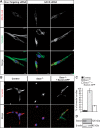
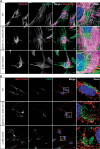
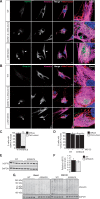
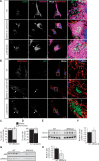
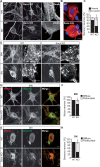
Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS) is caused by mutations in the gene SACS, encoding the 520 kDa protein sacsin. Although sacsin's physiological role is largely unknown, its sequence domains suggest a molecular chaperone or protein quality control function. Consequences of its loss include neurofilament network abnormalities, specifically accumulation and bundling of perikaryal and dendritic neurofilaments. To investigate if loss of sacsin affects intermediate filaments more generally, the distribution of vimentin was analysed in ARSACS patient fibroblasts and in cells where sacsin expression was reduced. Abnormal perinuclear accumulation of vimentin filaments, which sometimes had a cage-like appearance, occurred in sacsin-deficient cells. Mitochondria and other organelles were displaced to the periphery of vimentin accumulations. Reorganization of the vimentin network occurs in vitro under stress conditions, including when misfolded proteins accumulate. In ARSACS patient fibroblasts HSP70, ubiquitin and the autophagy-lysosome pathway proteins Lamp2 and p62 relocalized to the area of the vimentin accumulation. There was no overall increase in ubiquitinated proteins, suggesting the ubiquitin-proteasome system was not impaired. There was evidence for alterations in the autophagy-lysosome pathway. Specifically, in ARSACS HDFs cellular levels of Lamp2 were elevated while levels of p62, which is degraded in autophagy, were decreased. Moreover, autophagic flux was increased in ARSACS HDFs under starvation conditions. These data show that loss of sacsin effects the organization of intermediate filaments in multiple cell types, which impacts the cellular distribution of other organelles and influences autophagic activity.
© The Author 2017. Published by Oxford University Press.
Figures

References
-
- Bouhlal Y., Amouri R., El Euch-Fayeche G., Hentati F. (2011) Autosomal recessive spastic ataxia of Charlevoix-Saguenay: an overview. Parkinsonism Relat. Disord., 17, 418–422. - PubMed
-
- Girard M., Lariviere R., Parfitt D.A., Deane E.C., Gaudet R., Nossova N., Blondeau F., Prenosil G., Vermeulen E.G., Duchen M.R.. et al. (2012) Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS). Proc. Natl Acad. Sci. U. S. A., 109, 1661–1666. - PMC - PubMed
-
- Engert J.C., Berube P., Mercier J., Dore C., Lepage P., Ge B., Bouchard J.P., Mathieu J., Melancon S.B., Schalling M.. et al. (2000) ARSACS, a spastic ataxia common in northeastern Quebec, is caused by mutations in a new gene encoding an 11.5-kb ORF. NatGenet, 24, 120–125. - PubMed
-
- Anderson J.F., Siller E., Barral J.M. (2010) The sacsin repeating region (SRR): a novel Hsp90-related supra-domain associated with neurodegeneration. J. Mol. Biol., 400, 665–674. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

