Intergenerational impact of paternal lifetime exposures to both folic acid deficiency and supplementation on reproductive outcomes and imprinted gene methylation
- PMID: 28535307
- PMCID: PMC5909862
- DOI: 10.1093/molehr/gax029
Intergenerational impact of paternal lifetime exposures to both folic acid deficiency and supplementation on reproductive outcomes and imprinted gene methylation
Abstract
Study question: Do paternal exposures to folic acid deficient (FD), and/or folic acid supplemented (FS) diets, throughout germ cell development adversely affect male germ cells and consequently offspring health outcomes?
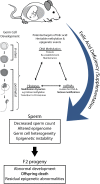
Summary answer: Male mice exposed over their lifetimes to both FD and FS diets showed decreased sperm counts and altered imprinted gene methylation with evidence of transmission of adverse effects to the offspring, including increased postnatal-preweaning mortality and variability in imprinted gene methylation.
What is known already: There is increasing evidence that disruptions in male germ cell epigenetic reprogramming are associated with offspring abnormalities and intergenerational disease. The fetal period is the critical time of DNA methylation pattern acquisition for developing male germ cells and an adequate supply of methyl donors is required. In addition, DNA methylation patterns continue to be remodeled during postnatal spermatogenesis. Previous studies have shown that lifetime (prenatal and postnatal) folic acid deficiency can alter the sperm epigenome and increase the incidence of fetal morphological abnormalities.
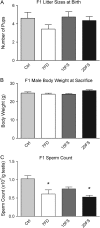
Study design, size, duration: Female BALB/c mice (F0) were placed on one of four amino-acid defined diets for 4 weeks before pregnancy and throughout pregnancy and lactation: folic acid control (Ctrl; 2 mg/kg), 7-fold folic acid deficient (7FD; 0.3 mg/kg), 10-fold high FS (10FS, 20 mg/kg) or 20-fold high FS (20FS, 40 mg/kg) diets. F1 males were weaned to their respective prenatal diets to allow for diet exposure during all windows of germline epigenetic reprogramming: the erasure, re-establishment and maintenance phases.
Participants/materials, settings, methods: F0 females were mated with chow-fed males to produce F1 litters whose germ cells were exposed to the diets throughout embryonic development. F1 males were subsequently mated with chow-fed female mice. Two F2 litters, unexposed to the experimental diets, were generated from each F1 male; one litter was collected at embryonic day (E)18.5 and one delivered and followed postnatally. DNA methylation at a global level and at the differentially methylated regions of imprinted genes (H19, Imprinted Maternally Expressed Transcript (Non-Protein Coding)-H19, Small Nuclear Ribonucleoprotein Polypeptide N-Snrpn, KCNQ1 Opposite Strand/Antisense Transcript 1 (Non-Protein Coding)-Kcnq1ot1, Paternally Expressed Gene 1-Peg1 and Paternally Expressed Gene 3-Peg3) was assessed by luminometric methylation analysis and bisulfite pyrosequencing, respectively, in F1 sperm, F2 E18.5 placenta and F2 E18.5 brain cortex.
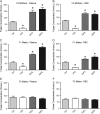
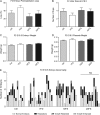
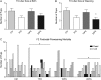
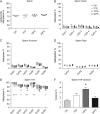
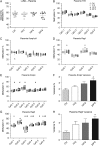
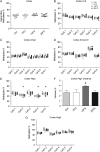
Main results and the role of chance: F1 males exhibited lower sperm counts following lifetime exposure to both folic acid deficiency and the highest dose of folic acid supplementation (20FS), (both P < 0.05). Post-implantation losses were increased amongst F2 E18.5 day litters from 20FS exposed F1 males (P < 0.05). F2 litters derived from both 7FD and 20FS exposed F1 males had significantly higher postnatal-preweaning pup death (both P < 0.05). Sperm from 10FS exposed males had increased variance in methylation across imprinted gene H19, P < 0.05; increased variance at a few sites within H19 was also found for the 7FD and 20FS groups (P < 0.05). While the 20FS diet resulted in inter-individual alterations in methylation across the imprinted genes Snrpn and Peg3 in F2 E18.5 placenta, ≥50% of individual sites tested in Peg1 and/or Peg3 were affected in the 7FD and 10FS groups. Inter-individual alterations in Peg1 methylation were found in F2 E18.5 day 10FS group brain cortex (P < 0.05).
Large scale data: Not applicable.
Limitations reasons for caution: The cause of the increase in postnatal-preweaning mortality was not investigated post-mortem. Further studies are required to understand the mechanisms underlying the adverse effects of folic acid deficiency and supplementation on developing male germ cells. Genome-wide DNA and histone methylome studies as well as gene expression studies are required to better understand the links between folic acid exposures, an altered germ cell epigenome and offspring outcomes.
Wider implications of the findings: The findings of this study provide further support for paternally transmitted environmental effects. The results indicate that both folic acid deficiency and high dose supplementation can be detrimental to germ cell development and reproductive fitness, in part by altering DNA methylation in sperm.
Study funding and competing interests: This study was supported by a grant to J.M.T. from the Canadian Institutes of Health Research (CIHR #89944). The authors declare they have no conflicts of interest.
Keywords: DNA methylation; developmental programming; epigenetics; folate; folic acid; intergenerational effects; male-mediated; paternal effects; sperm.
© The Author 2017. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com
Figures

References
-
- Bailey RL, Mills JL, Yetley EA, Gahche JJ, Pfeiffer CM, Dwyer JT, Dodd KW, Sempos CT, Betz JM, Picciano MF. Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged > or =60 y in the United States. Am J Clin Nutr 2010;92:383–389. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

