Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden
- PMID: 28535785
- PMCID: PMC5442667
- DOI: 10.1186/s13195-017-0263-9
Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden
Abstract
Background: The complexity of medication therapy in older adults with multiple comorbidities often leads to inappropriate prescribing. Drugs with anticholinergic properties are of particular interest because many are not recognized for this property; their use may lead to increased anticholinergic burden resulting in significant health risks, as well as negatively impacting cognition. Medication therapy management (MTM) interventions showed promise in addressing inappropriate medication use, but the effectiveness of targeted multidisciplinary team interventions addressing anticholinergic medications in older populations is yet to be determined.
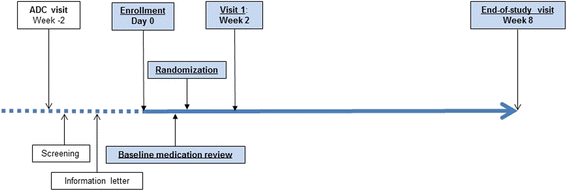
Methods: We conducted an 8-week, parallel-arm, randomized trial to evaluate whether a targeted patient-centered pharmacist-physician team MTM intervention ("targeted MTM intervention") reduced the use of inappropriate anticholinergic medications in older patients enrolled in a longitudinal cohort at University of Kentucky's Alzheimer's Disease Center. Study outcomes included changes in the medication appropriateness index (MAI) targeting anticholinergic medications and in the anticholinergic drug scale (ADS) score from baseline to the end of study.
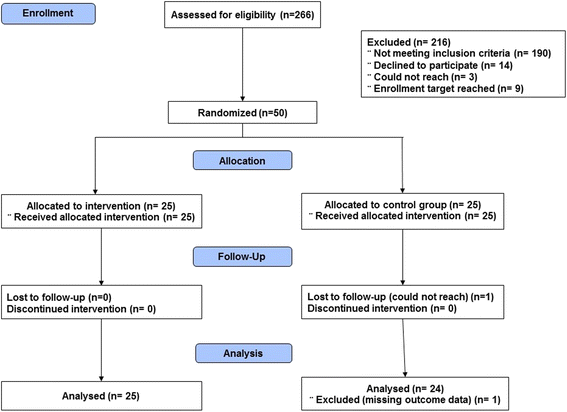
Results: Between October 1, 2014 and September 30, 2015 we enrolled and randomized 50 participants taking at least one medication with anticholinergic properties. Of these, 35 (70%) were women, 45 (90%) were white, and 33 (66%) were cognitively intact (clinical dementia rating [CDR] = 0); mean age was 77.7 ± 6.6 years. At baseline, the mean MAI was 12.6 ± 6.3; 25 (50%) of the participants used two or more anticholinergics, and the mean ADS score was 2.8 ± 1.6. After randomization, although no statistically significant difference was noted between groups, we identified a potentially meaningful imbalance as the intervention group had more participants with intact cognition, and thus included CDR in all of the analyses. The targeted MTM intervention resulted in statistically significant CDR adjusted differences between groups with regard to improved MAI (change score of 3.6 (1.1) for the MTM group as compared with 1.0 (0.9) for the control group, p = 0.04) and ADS (change score of 1.0 (0.3) for the MTM group as compared with 0.2 (0.3) for the control group, p = 0.03).
Conclusions: Our targeted MTM intervention resulted in improvement in anticholinergic medication appropriateness and reduced the use of inappropriate anticholinergic medications in older patients. Our results show promise in an area of great importance to ensure optimum outcomes for medications used in older adults.
Trial registration: ClinicalTrials.gov NCT02172612 . Registered 20 June 2014.
Keywords: Alzheimer’s Disease Center; Anticholinergic medication; Medication therapy management intervention; Older adults.
Figures
Similar articles
-
One-Year Evaluation of a Targeted Medication Therapy Management Intervention for Older Adults.J Manag Care Spec Pharm. 2020 Apr;26(4):520-528. doi: 10.18553/jmcp.2020.26.4.520. J Manag Care Spec Pharm. 2020. PMID: 32223601 Free PMC article.
-
Potentially inappropriate prescriptions of anticholinergics drugs in Alzheimer's disease patients.Geriatr Gerontol Int. 2019 Sep;19(9):913-917. doi: 10.1111/ggi.13748. Epub 2019 Jul 24. Geriatr Gerontol Int. 2019. PMID: 31342625
-
Acceptability of patient-centered, multi-disciplinary medication therapy management recommendations: results from the INCREASE randomized study.BMC Geriatr. 2023 Mar 10;23(1):137. doi: 10.1186/s12877-023-03876-4. BMC Geriatr. 2023. PMID: 36894900 Free PMC article. Clinical Trial.
-
Development of an Anticholinergic Burden Scale specific for Korean older adults.Geriatr Gerontol Int. 2019 Jul;19(7):628-634. doi: 10.1111/ggi.13680. Epub 2019 Apr 29. Geriatr Gerontol Int. 2019. PMID: 31033150
-
Are potentially inappropriate and anticholinergic medications being prescribed for institutionalized elderly subjects?Fundam Clin Pharmacol. 2020 Dec;34(6):743-748. doi: 10.1111/fcp.12560. Epub 2020 May 17. Fundam Clin Pharmacol. 2020. PMID: 32289182 Review.
Cited by
-
An investigation of new medications initiation during ambulatory care visits in patients with dementia.Explor Res Clin Soc Pharm. 2021 Aug 8;3:100058. doi: 10.1016/j.rcsop.2021.100058. eCollection 2021 Sep. Explor Res Clin Soc Pharm. 2021. PMID: 35480611 Free PMC article.
-
The Prognostic Utility of Anticholinergic Burden Scales: An Integrative Review and Gap Analysis.Drugs Aging. 2023 Sep;40(9):763-783. doi: 10.1007/s40266-023-01050-4. Epub 2023 Jul 18. Drugs Aging. 2023. PMID: 37462902
-
Pharmacist-driven interventions to de-escalate urinary antimuscarinics in the Programs of All-Inclusive Care for the Elderly.J Am Geriatr Soc. 2022 Nov;70(11):3230-3238. doi: 10.1111/jgs.17965. Epub 2022 Jul 28. J Am Geriatr Soc. 2022. PMID: 35900034 Free PMC article.
-
Determinants of Sleep Medication Use among Participants in the National Alzheimer's Coordinating Center Uniform Data Set.J Appl Gerontol. 2020 Dec;39(12):1340-1349. doi: 10.1177/0733464819888447. Epub 2019 Nov 20. J Appl Gerontol. 2020. PMID: 31747852 Free PMC article.
-
The Effect of Plan Type and Comprehensive Medication Reviews on High-Risk Medication Use.J Manag Care Spec Pharm. 2018 May;24(5):416-422. doi: 10.18553/jmcp.2018.24.5.416. J Manag Care Spec Pharm. 2018. PMID: 29694292 Free PMC article.
References
-
- Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007-2008 NCHS data brief. NCHS Data Brief. 2010;(42):1–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical