Fibrates for the treatment of cholestatic itch (FITCH): study protocol for a randomized controlled trial
- PMID: 28535810
- PMCID: PMC5442649
- DOI: 10.1186/s13063-017-1966-8
Fibrates for the treatment of cholestatic itch (FITCH): study protocol for a randomized controlled trial
Abstract
Background: Pruritus (itch) is a frequent, burdensome and difficult-to-treat symptom in patients with cholestasis. Fibrates are currently under investigation for the treatment of primary biliary cholangitis in patients with a suboptimal response to ursodeoxycholic acid. Moreover, there is empirical evidence for a possible antipruritic effect. We aim to prove this in a randomized controlled trial, including patients with cholestatic liver diseases other than primary biliary cholangitis that are accompanied by pruritus.
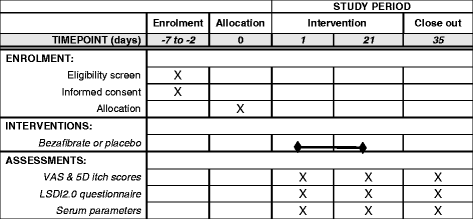
Methods: A multicenter investigator-initiated, double-blind, randomized placebo-controlled trial to evaluate the effect of bezafibrate on cholestatic pruritus in 84 adult patients with primary biliary cholangitis or primary/secondary sclerosing cholangitis. Primary outcome is the proportion of patients with a reduction of itch intensity of 50% or more (measured on a Visual Analog Scale) after 21 days of treatment with bezafibrate 400 mg qid or placebo. Secondary outcomes include the effect of bezafibrate on a five-dimensional itch score, liver disease-specific quality of life, serum liver tests and autotaxin activity. Safety will be evaluated through serum parameters for kidney function and rhabdomyolysis as well as precise recording of (serious) adverse events. We provide a schematic overview of the study protocol and describe the methods used to recruit and randomize patients, collect and handle data and perform statistical analyses.
Discussion: Given its favorable safety profile and anticholestatic properties, bezafibrate may become the new first-line treatment option for treating cholestatic pruritus.
Trial registration: Netherlands Trial Register, ID: NCT02701166 . Registered on 2 March 2016; Netherlands Trial Register, ID: NTR5436 . Registered on 3 August 2015.
Keywords: Bezafibrate; Itch; Primary biliary cholangitis; Primary sclerosing cholangitis; Pruritus; Secondary sclerosing cholangitis.
Figures
Similar articles
-
Fibrates for Itch (FITCH) in Fibrosing Cholangiopathies: A Double-Blind, Randomized, Placebo-Controlled Trial.Gastroenterology. 2021 Feb;160(3):734-743.e6. doi: 10.1053/j.gastro.2020.10.001. Epub 2020 Oct 5. Gastroenterology. 2021. PMID: 33031833 Clinical Trial.
-
Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study.Lancet. 2017 Mar 18;389(10074):1114-1123. doi: 10.1016/S0140-6736(17)30319-7. Epub 2017 Feb 8. Lancet. 2017. PMID: 28187915 Clinical Trial.
-
Use of Butorphanol as Treatment for Cholestatic Itch.Dig Dis Sci. 2021 May;66(5):1693-1699. doi: 10.1007/s10620-020-06392-2. Epub 2020 Jun 18. Dig Dis Sci. 2021. PMID: 32556969
-
Pharmacological Therapy of Pruritus in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.J Clin Gastroenterol. 2023 Feb 1;57(2):143-152. doi: 10.1097/MCG.0000000000001797. J Clin Gastroenterol. 2023. PMID: 36598806
-
Cholestatic Pruritus Treatments in Primary Biliary Cholangitis and Primary Sclerosing Cholangitis: A Systematic Literature Review.Dig Dis Sci. 2023 Jun;68(6):2710-2730. doi: 10.1007/s10620-023-07862-z. Epub 2023 Mar 18. Dig Dis Sci. 2023. PMID: 36933112 Free PMC article.
Cited by
-
Efficacy of Treatments for Cholestatic Pruritus: A Systemic Review and Meta-analysis.Acta Derm Venereol. 2022 Feb 22;102:adv00653. doi: 10.2340/actadv.v102.310. Acta Derm Venereol. 2022. PMID: 35088869 Free PMC article.
-
Primary Biliary Cholangitis and Primary Sclerosing Cholangitis: Current Knowledge of Pathogenesis and Therapeutics.Biomedicines. 2022 May 31;10(6):1288. doi: 10.3390/biomedicines10061288. Biomedicines. 2022. PMID: 35740310 Free PMC article. Review.
-
The Simple Cholestatic Complaints Score is a valid and quick patient-reported outcome measure in primary sclerosing cholangitis.Liver Int. 2020 Nov;40(11):2758-2766. doi: 10.1111/liv.14644. Liver Int. 2020. PMID: 32841496 Free PMC article.
-
Current understanding of primary biliary cholangitis.Clin Mol Hepatol. 2021 Jan;27(1):1-21. doi: 10.3350/cmh.2020.0028. Epub 2020 Dec 3. Clin Mol Hepatol. 2021. PMID: 33264835 Free PMC article. Review.
-
Cholestatic pruritus: Emerging mechanisms and therapeutics.J Am Acad Dermatol. 2019 Dec;81(6):1371-1378. doi: 10.1016/j.jaad.2019.04.035. Epub 2019 Apr 19. J Am Acad Dermatol. 2019. PMID: 31009666 Free PMC article.
References
-
- Miyaguchi S, Ebinuma H, Imaeda H, Nitta Y, Watanabe T, Saito H, et al. A novel treatment for refractory primary biliary cirrhosis? Hepatogastroenterology. 2000;47:1518–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials