Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: towards an intelligent knife for breast cancer surgery
- PMID: 28535818
- PMCID: PMC5442854
- DOI: 10.1186/s13058-017-0845-2
Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: towards an intelligent knife for breast cancer surgery
Abstract
Background: Re-operation for positive resection margins following breast-conserving surgery occurs frequently (average = 20-25%), is cost-inefficient, and leads to physical and psychological morbidity. Current margin assessment techniques are slow and labour intensive. Rapid evaporative ionisation mass spectrometry (REIMS) rapidly identifies dissected tissues by determination of tissue structural lipid profiles through on-line chemical analysis of electrosurgical aerosol toward real-time margin assessment.
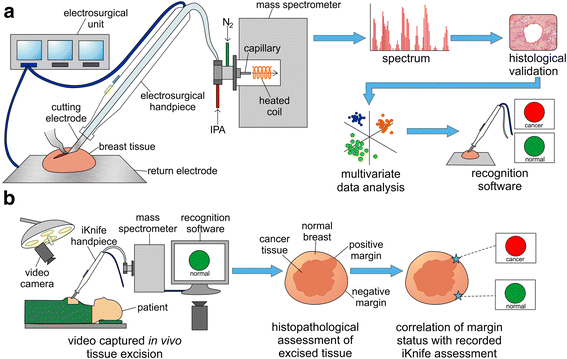
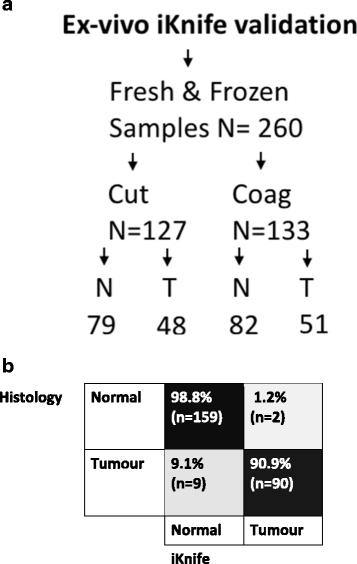
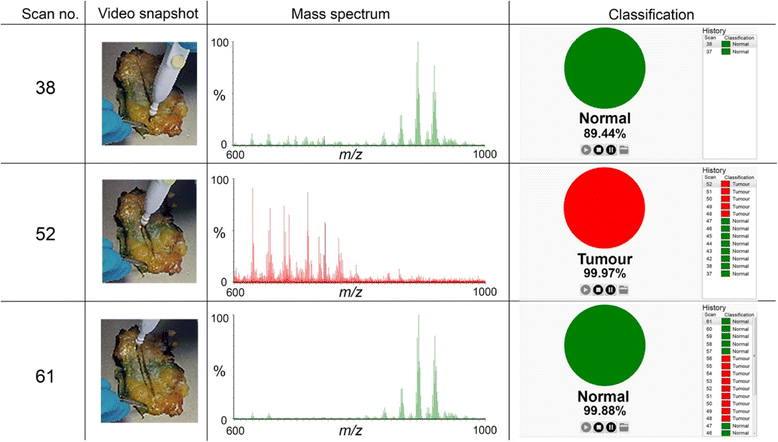
Methods: Electrosurgical aerosol produced from ex-vivo and in-vivo breast samples was aspirated into a mass spectrometer (MS) using a monopolar hand-piece. Tissue identification results obtained by multivariate statistical analysis of MS data were validated by histopathology. Ex-vivo classification models were constructed from a mass spectral database of normal and tumour breast samples. Univariate and tandem MS analysis of significant peaks was conducted to identify biochemical differences between normal and cancerous tissues. An ex-vivo classification model was used in combination with bespoke recognition software, as an intelligent knife (iKnife), to predict the diagnosis for an ex-vivo validation set. Intraoperative REIMS data were acquired during breast surgery and time-synchronized to operative videos.
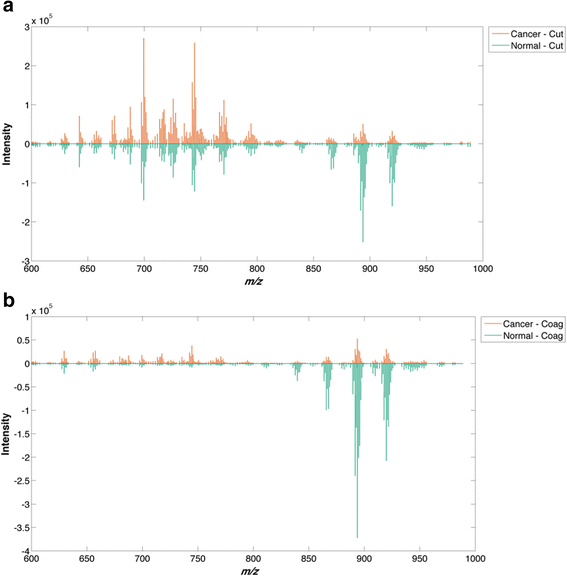
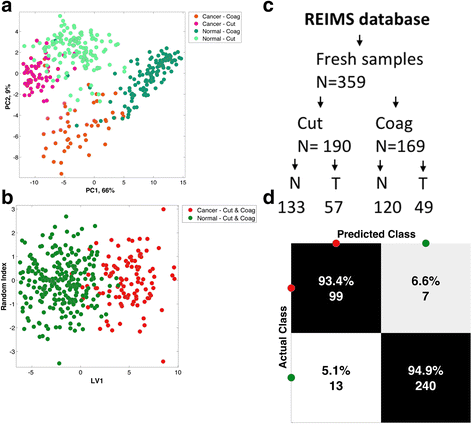
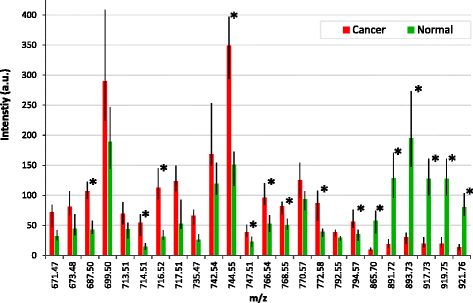
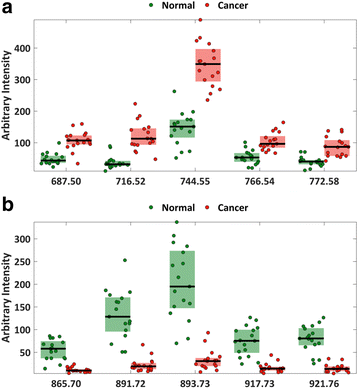
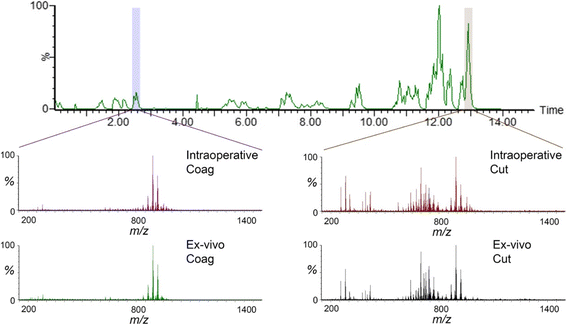
Results: A classification model using histologically validated spectral data acquired from 932 sampling points in normal tissue and 226 in tumour tissue provided 93.4% sensitivity and 94.9% specificity. Tandem MS identified 63 phospholipids and 6 triglyceride species responsible for 24 spectral differences between tissue types. iKnife recognition accuracy with 260 newly acquired fresh and frozen breast tissue specimens (normal n = 161, tumour n = 99) provided sensitivity of 90.9% and specificity of 98.8%. The ex-vivo and intra-operative method produced visually comparable high intensity spectra. iKnife interpretation of intra-operative electrosurgical vapours, including data acquisition and analysis was possible within a mean of 1.80 seconds (SD ±0.40).
Conclusions: The REIMS method has been optimised for real-time iKnife analysis of heterogeneous breast tissues based on subtle changes in lipid metabolism, and the results suggest spectral analysis is both accurate and rapid. Proof-of-concept data demonstrate the iKnife method is capable of online intraoperative data collection and analysis. Further validation studies are required to determine the accuracy of intra-operative REIMS for oncological margin assessment.
Keywords: Breast; Cancer; Intelligent knife; Intraoperative margin assessment; Margins; Mass spectrometry; REIMS; Rapid evaporative ionisation mass spectrometry; Surgery; iKnife.
Figures
References
-
- Stewart BW, Wild C. World Cancer Report 2014. International Agency for Research on Cancer; 2014. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports....
-
- Jeevan R, Cromwell DA, Trivella M, Lawrence G, Kearins O, Pereira J, Sheppard C, Caddy CM, van der Meulen JHP. Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ. 2012;345:e4505. http://www.bmj.com/content/345/bmj.e4505. - PMC - PubMed
-
- Wilke LG, Czechura T, Wang C, Lapin B, Liederbach E, Winchester DP, Yao K. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004-2010. JAMA Surg. 2014;149(12):1296–305. doi: 10.1001/jamasurg.2014.926. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

