Development of a Modular Research Platform to Create Medical Observational Studies for Mobile Devices
- PMID: 28536095
- PMCID: PMC5461422
- DOI: 10.2196/resprot.7705
Development of a Modular Research Platform to Create Medical Observational Studies for Mobile Devices
Abstract
Background: Observational studies have proven to be a valuable resource in medical research, especially when performed on a large scale. Recently, mobile device-based observational studies have been discovered by an increasing number of researchers as a promising new source of information. However, the development and deployment of app-based studies is not trivial and requires profound programming skills.
Objective: The aim of this project was to develop a modular online research platform that allows researchers to create medical studies for mobile devices without extensive programming skills.
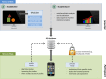
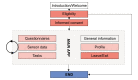
Methods: The platform approach for a modular research platform consists of three major components. A Web-based platform forms the researchers' main workplace. This platform communicates via a shared database with a platform independent mobile app. Furthermore, a separate Web-based login platform for physicians and other health care professionals is outlined and completes the concept.
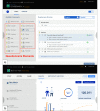
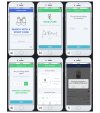
Results: A prototype of the research platform has been developed and is currently in beta testing. Simple questionnaire studies can be created within minutes and published for testing purposes. Screenshots of an example study are provided, and the general working principle is displayed.
Conclusions: In this project, we have created a basis for a novel research platform. The necessity and implications of a modular approach were displayed and an outline for future development given. International researchers are invited and encouraged to participate in this ongoing project.
Keywords: app-based study; mHealth; mobile health; research platform; telemedicine.
©Martin Zens, Birgit Grotejohann, Adrian Tassoni, Fabian Duttenhoefer, Norbert P Südkamp, Philipp Niemeyer. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 23.05.2017.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- Best S, Krueger B, Hubbard C, Smith A. An Assessment of the Generalizability of Internet Surveys. Social Science Computer Review. 2001 May 01;19(2):131–145. doi: 10.1177/089443930101900201. doi: 10.1177/089443930101900201. - DOI
-
- Bandilla W, Bosnjak M, Altdorfer P. Survey Administration Effects?: A Comparison of Web-Based and Traditional Written Self-Administered Surveys Using the ISSP Environment Module. Social Science Computer Review. 2003 May 01;21(2):235–243. doi: 10.1177/0894439303021002009. doi: 10.1177/0894439303021002009. - DOI
-
- Braithwaite D, Emery J, De Lusignan S, Sutton S. Using the Internet to conduct surveys of health professionals: a valid alternative? Fam Pract. 2003 Oct;20(5):545–551. - PubMed
-
- Webster DE, Suver C, Doerr M, Mounts E, Domenico L, Petrie T, Leachman SA, Trister AD, Bot BM. The Mole Mapper Study, mobile phone skin imaging and melanoma risk data collected using ResearchKit. Sci Data. 2017 Feb 14;4:170005. doi: 10.1038/sdata.2017.5. doi: 10.1038/sdata.2017.5. - DOI - PMC - PubMed
-
- BinDhim NF, Alanazi EM, Aljadhey H, Basyouni MH, Kowalski SR, Pont LG, Shaman AM, Trevena L, Alhawassi TM. Does a Mobile Phone Depression-Screening App Motivate Mobile Phone Users With High Depressive Symptoms to Seek a Health Care Professional's Help? J Med Internet Res. 2016 Jun 27;18(6):e156. doi: 10.2196/jmir.5726. doi: 10.2196/jmir.5726. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

