Randomized Controlled Trial of Mineralocorticoid Receptor Blockade in Children with Chronic Kidney Allograft Nephropathy
- PMID: 28536123
- PMCID: PMC5544507
- DOI: 10.2215/CJN.05300516
Randomized Controlled Trial of Mineralocorticoid Receptor Blockade in Children with Chronic Kidney Allograft Nephropathy
Abstract
Background and objectives: We showed that mineralocorticoid receptor blockade (MRB) prevented acute and chronic cyclosporine nephropathy (CsA-Nx) in the rat. The aim of this translational study was to investigate the effect of long-term eplerenone administration on renal allograft function in children with biopsy-proven chronic allograft nephropathy (CAN).
Design, setting, participants, & measurements: Renal transplant children <18 years, biopsy-proven CAN, and a GFR>40 ml/min per 1.73 m2 were included. Patients with BK virus active nephritis, recurrence of renal disease, GFR decline in previous 3 months, or treated with calcium antagonists or antifungal drugs were excluded. They were randomized to receive placebo (n=10) or eplerenone 25 mg/d for 24 months (n=13). Visits were scheduled at baseline, 6, 12, and 24 months. At each period, a complete clinical examination was performed and blood and urine samples were taken. Urine creatinine, 8-hydroxylated-guanosine, heat shock protein 72 (HSP72), and kidney injury molecule (KIM-1) levels were also assessed. In kidney biopsy samples, the tubulo-interstitial area affected by fibrosis (TIF) and glomerulosclerosis were measured at baseline and after 24 months.
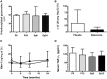
Results: The baseline eGFR was 80±6 in the placebo and 86±6 ml/min per 1.73 m2 in the eplerenone group; at 24 months it was 66±8 and 81±7 ml/min per 1.73 m2, respectively (P=0.33; 95% confidence intervals, -18 to 33 at baseline, and -11 to 40 after 24 months). The albumin-to-creatinine ratio was 110±74 in the placebo, and 265±140 mg/g in the eplerenone group; and after 24 months it was 276±140 and 228±88 mg/g, respectively (P=0.15; 95% confidence intervals, -283 to 593, and -485 to 391, respectively). In addition, the placebo exhibited a greater TIF, glomerulosclerosis, and urinary HSP72 compared with the eplerenone group.
Conclusions: Although this study was underpowered to provide definitive evidence that long-term eplerenone administration attenuates the progression of CAN in pediatric transplant patients, it encourages testing the potential benefit of MRB in this pediatric population.
Copyright © 2017 by the American Society of Nephrology.
Figures





References
-
- Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 - PubMed
-
- Haas M: Chronic allograft nephropathy or interstitial fibrosis and tubular atrophy: What is in a name? Curr Opin Nephrol Hypertens 23: 245–250, 2014 - PubMed
-
- Stegall MD, Park WD, Larson TS, Gloor JM, Cornell LD, Sethi S, Dean PG, Prieto M, Amer H, Textor S, Schwab T, Cosio FG: The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant 11: 698–707, 2011 - PubMed
-
- Viero RM, da Silva MG, dos Santos DC, de Carvalho MF, de Andrade LG: The role of renin-angiotensin system in the chronic allograft nephropathy: An immunohistochemical study. Ren Fail 37: 827–834, 2015 - PubMed
-
- Abramowicz D, Wissing KM, Broeders N: Nephrotoxicity of calcineurin inhibitors: New therapeutic approaches. Transplant Proc 32[1A Suppl]: 3S–5S, 2000 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

