The NLRP3 inflammasome contributes to sarcopenia and lower muscle glycolytic potential in old mice
- PMID: 28536183
- PMCID: PMC5582883
- DOI: 10.1152/ajpendo.00060.2017
The NLRP3 inflammasome contributes to sarcopenia and lower muscle glycolytic potential in old mice
Abstract
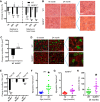
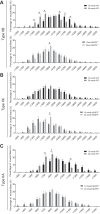
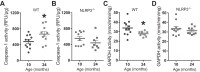
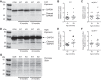
The mechanisms underpinning decreased skeletal muscle strength and slowing of movement during aging are ill-defined. "Inflammaging," increased inflammation with advancing age, may contribute to aspects of sarcopenia, but little is known about the participatory immune components. We discovered that aging was associated with increased caspase-1 activity in mouse skeletal muscle. We hypothesized that the caspase-1-containing NLRP3 inflammasome contributes to sarcopenia in mice. Male C57BL/6J wild-type (WT) and NLRP3-/- mice were aged to 10 (adult) and 24 mo (old). NLRP3-/- mice were protected from decreased muscle mass (relative to body mass) and decreased size of type IIB and IIA myofibers, which occurred between 10 and 24 mo of age in WT mice. Old NLRP3-/- mice also had increased relative muscle strength and endurance and were protected from age-related increases in the number of myopathic fibers. We found no evidence of age-related or NLRP3-dependent changes in markers of systemic inflammation. Increased caspase-1 activity was associated with GAPDH proteolysis and reduced GAPDH enzymatic activity in skeletal muscles from old WT mice. Aging did not alter caspase-1 activity, GAPDH proteolysis, or GAPDH activity in skeletal muscles of NLRP3-/- mice. Our results show that the NLRP3 inflammasome participates in age-related loss of muscle glycolytic potential. Deletion of NLRP3 mitigates both the decline in glycolytic myofiber size and the reduced activity of glycolytic enzymes in muscle during aging. We propose that the etiology of sarcopenia involves direct communication between immune responses and metabolic flux in skeletal muscle.
Keywords: NOD-like receptor family pyrin domain containing 3; aging; caspase; glycolysis; inflammation; muscle.
Copyright © 2017 the American Physiological Society.
Figures

Similar articles
-
Lack of NLRP3 Inflammasome Activation Reduces Age-Dependent Sarcopenia and Mitochondrial Dysfunction, Favoring the Prophylactic Effect of Melatonin.J Gerontol A Biol Sci Med Sci. 2019 Oct 4;74(11):1699-1708. doi: 10.1093/gerona/glz079. J Gerontol A Biol Sci Med Sci. 2019. PMID: 30869745
-
Implication of the NLRP3 Inflammasome in Bovine Age-Related Sarcopenia.Int J Mol Sci. 2021 Mar 30;22(7):3609. doi: 10.3390/ijms22073609. Int J Mol Sci. 2021. PMID: 33808510 Free PMC article.
-
Increased Expression of the NOD-like Receptor Family, Pyrin Domain Containing 3 Inflammasome in Dermatomyositis and Polymyositis is a Potential Contributor to Their Pathogenesis.Chin Med J (Engl). 2016 May 5;129(9):1047-52. doi: 10.4103/0366-6999.180528. Chin Med J (Engl). 2016. PMID: 27098789 Free PMC article.
-
New insights into the function of the NLRP3 inflammasome in sarcopenia: mechanism and therapeutic strategies.Metabolism. 2024 Sep;158:155972. doi: 10.1016/j.metabol.2024.155972. Epub 2024 Jul 6. Metabolism. 2024. PMID: 38972476 Review.
-
Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease.Am J Respir Cell Mol Biol. 2016 Feb;54(2):151-60. doi: 10.1165/rcmb.2015-0231TR. Am J Respir Cell Mol Biol. 2016. PMID: 26418144 Free PMC article. Review.
Cited by
-
Pyroptosis and Sarcopenia: Frontier Perspective of Disease Mechanism.Cells. 2022 Mar 23;11(7):1078. doi: 10.3390/cells11071078. Cells. 2022. PMID: 35406642 Free PMC article. Review.
-
Metabolic regulation of inflammasomes in inflammation.Immunology. 2019 Jun;157(2):95-109. doi: 10.1111/imm.13056. Epub 2019 Apr 8. Immunology. 2019. PMID: 30851192 Free PMC article. Review.
-
Anticytokine Agents: Targeting Interleukin Signaling Pathways for the Treatment of Atherothrombosis.Circ Res. 2019 Feb;124(3):437-450. doi: 10.1161/CIRCRESAHA.118.313129. Circ Res. 2019. PMID: 30702995 Free PMC article. Review.
-
The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy.Diabetes Metab Syndr Obes. 2023 May 30;16:1541-1554. doi: 10.2147/DMSO.S410834. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 37275941 Free PMC article. Review.
-
Impact of Melatonin on Skeletal Muscle and Exercise.Cells. 2020 Jan 24;9(2):288. doi: 10.3390/cells9020288. Cells. 2020. PMID: 31991655 Free PMC article. Review.
References
-
- Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KHG, Masters SL, Schroder K, Cooper MA, O’Neill LAJ. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21: 248–255, 2015. doi: 10.1038/nm.3806. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

