Understanding the essential proton-pumping kinetic gates and decoupling mutations in cytochrome c oxidase
- PMID: 28536198
- PMCID: PMC5468613
- DOI: 10.1073/pnas.1703654114
Understanding the essential proton-pumping kinetic gates and decoupling mutations in cytochrome c oxidase
Abstract
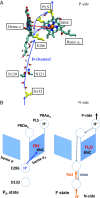
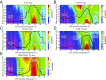
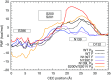
Cytochrome c oxidase (CcO) catalyzes the reduction of oxygen to water and uses the released free energy to pump protons against the transmembrane proton gradient. To better understand the proton-pumping mechanism of the wild-type (WT) CcO, much attention has been given to the mutation of amino acid residues along the proton translocating D-channel that impair, and sometimes decouple, proton pumping from the chemical catalysis. Although their influence has been clearly demonstrated experimentally, the underlying molecular mechanisms of these mutants remain unknown. In this work, we report multiscale reactive molecular dynamics simulations that characterize the free-energy profiles of explicit proton transport through several important D-channel mutants. Our results elucidate the mechanisms by which proton pumping is impaired, thus revealing key kinetic gating features in CcO. In the N139T and N139C mutants, proton back leakage through the D-channel is kinetically favored over proton pumping due to the loss of a kinetic gate in the N139 region. In the N139L mutant, the bulky L139 side chain inhibits timely reprotonation of E286 through the D-channel, which impairs both proton pumping and the chemical reaction. In the S200V/S201V double mutant, the proton affinity of E286 is increased, which slows down both proton pumping and the chemical catalysis. This work thus not only provides insight into the decoupling mechanisms of CcO mutants, but also explains how kinetic gating in the D-channel is imperative to achieving high proton-pumping efficiency in the WT CcO.
Keywords: cytochrome c oxidase; decoupling mutants; multiscale; proton pump; proton transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wikstrom MKF. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977;266:271–273. - PubMed
-
- Ferguson-Miller S, Babcock GT. Heme/copper terminal oxidases. Chem Rev. 1996;96:2889–2908. - PubMed
-
- Kaila VR, Verkhovsky MI, Wikström M. Proton-coupled electron transfer in cytochrome oxidase. Chem Rev. 2010;110:7062–7081. - PubMed
-
- Konstantinov AA, Siletsky S, Mitchell D, Kaulen A, Gennis RB. The roles of the two proton input channels in cytochrome c oxidase from Rhodobacter sphaeroides probed by the effects of site-directed mutations on time-resolved electrogenic intraprotein proton transfer. Proc Natl Acad Sci USA. 1997;94:9085–9090. - PMC - PubMed
-
- Wikström M, Jasaitis A, Backgren C, Puustinen A, Verkhovsky MI. The role of the D- and K-pathways of proton transfer in the function of the haem-copper oxidases. Biochim Biophys Acta. 2000;1459:514–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

