Functional role of the three conserved cysteines in the N domain of visual arrestin-1
- PMID: 28536260
- PMCID: PMC5535024
- DOI: 10.1074/jbc.M117.790386
Functional role of the three conserved cysteines in the N domain of visual arrestin-1
Abstract
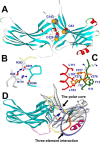
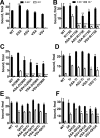
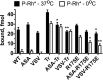
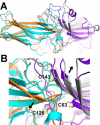
Arrestins specifically bind active and phosphorylated forms of their cognate G protein-coupled receptors, blocking G protein coupling and often redirecting the signaling to alternative pathways. High-affinity receptor binding is accompanied by two major structural changes in arrestin: release of the C-tail and rotation of the two domains relative to each other. The first requires detachment of the arrestin C-tail from the body of the molecule, whereas the second requires disruption of the network of charge-charge interactions at the interdomain interface, termed the polar core. These events can be facilitated by mutations destabilizing the polar core or the anchoring of the C-tail that yield "preactivated" arrestins that bind phosphorylated and unphosphorylated receptors with high affinity. Here we explored the functional role in arrestin activation of the three native cysteines in the N domain, which are conserved in all arrestin subtypes. Using visual arrestin-1 and rhodopsin as a model, we found that substitution of these cysteines with serine, alanine, or valine virtually eliminates the effects of the activating polar core mutations on the binding to unphosphorylated rhodopsin while only slightly reducing the effects of the C-tail mutations. Thus, these three conserved cysteines play a role in the domain rotation but not in the C-tail release.
Keywords: activation; arrestin; conformational change; cysteines; photoreceptor; phototransduction; rhodopsin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Sullivan L. S., Bowne S. J., Koboldt D. C., Cadena E. L., Heckenlively J. R., Branham K. E., Birch D. G., Wheaton D. H., Jones K. D., Ruiz R. S., Pennesi M. E., Yang P., Davis-Boozer D., Northrup H., Gurevich V. V., et al. (2017) A novel dominant mutation in SAG, the Arrestin-1 gene, is a common cause of retinitis pigmentosa in Hispanic families in the southwestern United States. Invest. Ophthalmol. Vis. Sci. 58, 2774–2784 - PMC - PubMed
-
- Granzin J., Wilden U., Choe H. W., Labahn J., Krafft B., and Büldt G. (1998) X-ray crystal structure of arrestin from bovine rod outer segments. Nature 391, 918–921 - PubMed
-
- Hirsch J. A., Schubert C., Gurevich V. V., and Sigler P. B. (1999) The 2.8 A crystal structure of visual arrestin: a model for arrestin's regulation. Cell 97, 257–269 - PubMed
-
- Han M., Gurevich V. V., Vishnivetskiy S. A., Sigler P. B., and Schubert C. (2001) Crystal structure of β-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure 9, 869–880 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

