Coconut (Cocos nucifera) Ethanolic Leaf Extract Reduces Amyloid-β (1-42) Aggregation and Paralysis Prevalence in Transgenic Caenorhabditis elegans Independently of Free Radical Scavenging and Acetylcholinesterase Inhibition
- PMID: 28536360
- PMCID: PMC5489803
- DOI: 10.3390/biomedicines5020017
Coconut (Cocos nucifera) Ethanolic Leaf Extract Reduces Amyloid-β (1-42) Aggregation and Paralysis Prevalence in Transgenic Caenorhabditis elegans Independently of Free Radical Scavenging and Acetylcholinesterase Inhibition
Abstract
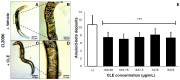
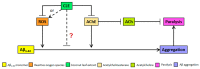
Virgin coconut oil (VCO) has been the subject of several studies which have aimed to alleviate Alzheimer's disease (AD) pathology, focusing on in vitro antioxidant and acetylcholinesterase (AChE) inhibitory activities. Here, we studied an underutilized and lesser-valued part of the coconut tree, specifically the leaves, using in vitro and in vivo approaches. Coconut leaf extract (CLE) was screened for antioxidant and AChE inhibitory properties in vitro and therapeutic effects in two strains of transgenic Caenorhabditis elegans expressing amyloid-β1-42 (Aβ1-42) in muscle cells. CLE demonstrated free radical scavenging activity with an EC50 that is 79-fold less compared to ascorbic acid, and an AChE inhibitory activity that is 131-fold less compared to Rivastigmine. Surprisingly, in spite of its low antioxidant activity and AChE inhibition, CLE reduced Aβ deposits by 30.31% in CL2006 in a dose-independent manner, and reduced the percentage of paralyzed nematodes at the lowest concentration of CLE (159.38 μg/mL), compared to dH₂O/vehicle (control). Phytochemical analysis detected glycosides, anthocyanins, and hydrolyzable tannins in CLE, some of which are known to be anti-amyloidogenic. Taken together, these findings suggest that CLE metabolites alternatively decrease AB1-42 aggregation and paralysis prevalence independently of free radical scavenging and AChE inhibition, and this warrants further investigation on the bioactive compounds of CLE.
Keywords: Alzheimer’s disease (AD); Caenorhabditis elegans; Cocos nucifera; coconut leaf extract; sporadic inclusion body myositis (sIBM).
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures


Similar articles
-
Exploring the therapeutic potential of Nelumbo nucifera leaf extract against amyloid-beta-induced toxicity in the Caenorhabditis elegans model of Alzheimer's disease.Front Pharmacol. 2024 Jun 24;15:1408031. doi: 10.3389/fphar.2024.1408031. eCollection 2024. Front Pharmacol. 2024. PMID: 38983916 Free PMC article.
-
6-Methyluracil derivatives as acetylcholinesterase inhibitors for treatment of Alzheimer's disease.Int J Risk Saf Med. 2015;27 Suppl 1:S69-71. doi: 10.3233/JRS-150694. Int J Risk Saf Med. 2015. PMID: 26639718
-
In vitro acetylcholinesterase inhibitory activity and the antioxidant properties of Aegle marmelos leaf extract: implications for the treatment of Alzheimer's disease.Psychogeriatrics. 2014 Mar;14(1):1-10. doi: 10.1111/psyg.12031. Psychogeriatrics. 2014. PMID: 24646308
-
The role of dietary coconut for the prevention and treatment of Alzheimer's disease: potential mechanisms of action.Br J Nutr. 2015 Jul 14;114(1):1-14. doi: 10.1017/S0007114515001452. Epub 2015 May 22. Br J Nutr. 2015. PMID: 25997382 Review.
-
Management of oxidative stress and other pathologies in Alzheimer's disease.Arch Toxicol. 2019 Sep;93(9):2491-2513. doi: 10.1007/s00204-019-02538-y. Epub 2019 Aug 22. Arch Toxicol. 2019. PMID: 31440798 Review.
Cited by
-
Analysis of the antiparasitic and anticancer activity of the coconut palm (Cocos nucifera L. ARECACEAE) from the natural reserve of Punta Patiño, Darién.PLoS One. 2019 Apr 2;14(4):e0214193. doi: 10.1371/journal.pone.0214193. eCollection 2019. PLoS One. 2019. PMID: 30939131 Free PMC article.
-
Natural Bioactive Products and Alzheimer's Disease Pathology: Lessons from Caenorhabditis elegans Transgenic Models.Diseases. 2022 May 13;10(2):28. doi: 10.3390/diseases10020028. Diseases. 2022. PMID: 35645249 Free PMC article. Review.
-
Molecular interactions with redox sites and salt bridges modulate the anti-aggregatory effect of flavonoid, tannin and cardenolide moieties against amyloid-beta (1-42) in silico.In Silico Pharmacol. 2017 Oct 13;5:11. doi: 10.1007/s40203-017-0033-1. eCollection 2017. In Silico Pharmacol. 2017. PMID: 29085768 Free PMC article.
-
Antiplasmodial activity of Cocos nucifera leaves in Plasmodium berghei-infected mice.J Parasit Dis. 2020 Jun;44(2):305-313. doi: 10.1007/s12639-020-01207-7. Epub 2020 Mar 9. J Parasit Dis. 2020. PMID: 32499668 Free PMC article.
-
Natural Compounds for Alzheimer's Disease Therapy: A Systematic Review of Preclinical and Clinical Studies.Int J Mol Sci. 2019 May 10;20(9):2313. doi: 10.3390/ijms20092313. Int J Mol Sci. 2019. PMID: 31083327 Free PMC article.
References
-
- Guo J.L., Narasimhan S., Changolkar L., He Z., Stieber A., Zhang B., Gathagan R.J., Iba M., McBride J.D., Trojanowski J.Q., et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 2016;213:1–20. doi: 10.1084/jem.20160833. - DOI - PMC - PubMed
-
- Nilson A.N., English K.C., Gerson J.E., Whittle T.B., Crain C.N., Xue J., Sengupta U., Castillo-Carranza D.L., Zhang W., Gupta P., et al. Tau Oligomers Associate with Inflammation in the Brain and Retina of Tauopathy Mice and in Neurodegenerative Diseases. J. Alzheimer’s Dis. 2016;55:1083–1099. doi: 10.3233/JAD-160912. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

