Synthesis and Preliminary Biological Evaluations of Fluorescent or 149Promethium Labeled Trastuzumab-Polyethylenimine
- PMID: 28536369
- PMCID: PMC5344248
- DOI: 10.3390/biomedicines4010001
Synthesis and Preliminary Biological Evaluations of Fluorescent or 149Promethium Labeled Trastuzumab-Polyethylenimine
Abstract
Background: Radioimmunotherapy utilize a targeting antibody coupled to a therapeutic isotope to target and treat a tumor or disease. In this study we examine the synthesis and cell binding of a polymer scaffold containing a radiotherapeutic isotope and a targeting antibody.
Methods: The multistep synthesis of a fluorescent or 149Promethium-labeled Trastuzumab-polyethyleneimine (PEI), Trastuzumab, or PEI is described. In vitro uptake, internalization and/or the binding affinity to the Her2/neu expressing human breast adenocarcinoma SKBr3 cells was investigated with the labeled compounds.
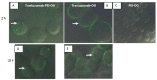
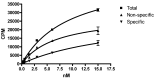
Results: Fluorescent-labeled Trastuzumab-PEI was internalized more into cells at 2 and 18 h than fluorescent-labeled Trastuzumab or PEI. The fluorescent-labeled Trastuzumab was concentrated on the cell surface at 2 and 18 h and the labeled PEI had minimal uptake. DOTA-PEI was prepared and contained an average of 16 chelates per PEI; the compound was radio-labeled with 149Promethium and conjugated to Trastuzumab. The purified 149Pm-DOTA-PEI-Trastuzumab had a radiochemical purity of 96.7% and a specific activity of 0.118 TBq/g. The compound demonstrated a dissociation constant for the Her2/neu receptor of 20.30 ± 6.91 nM.
Conclusion: The results indicate the DOTA-PEI-Trastuzumab compound has potential as a targeted therapeutic carrier, and future in vivo studies should be performed.
Keywords: Actinium-225; Promethium-149; Trastuzumab; polyethylenimine; radiotherapy; targeted therapy.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures



Similar articles
-
Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients.Int J Cancer. 2017 Feb 15;140(4):938-947. doi: 10.1002/ijc.30500. Epub 2016 Nov 18. Int J Cancer. 2017. PMID: 27813061 Clinical Trial.
-
Evaluation of an Anti-HER2 Nanobody Labeled with 225Ac for Targeted α-Particle Therapy of Cancer.Mol Pharm. 2018 Apr 2;15(4):1457-1466. doi: 10.1021/acs.molpharmaceut.7b00985. Epub 2018 Mar 7. Mol Pharm. 2018. PMID: 29502411
-
Targeted actinium-225 in vivo generators for therapy of ovarian cancer.Cancer Res. 2003 Aug 15;63(16):5084-90. Cancer Res. 2003. PMID: 12941838
-
In Vitro Assessment of 177Lu-Labeled Trastuzumab-Targeted Mesoporous Carbon@Silica Nanostructure for the Treatment of HER2-Positive Breast Cancer.Pharmaceuticals (Basel). 2024 Jun 5;17(6):732. doi: 10.3390/ph17060732. Pharmaceuticals (Basel). 2024. PMID: 38931400 Free PMC article.
-
Promethium: To Strive, to Seek, to Find and Not to Yield.Front Chem. 2020 Jul 10;8:588. doi: 10.3389/fchem.2020.00588. eCollection 2020. Front Chem. 2020. PMID: 32754576 Free PMC article. Review.
References
-
- National Research Council (US) and Institute of Medicine (US) Committee on State of the Science of Nuclear Medicine . Advancing Nuclear Medicine through Innovation. National Academies Press; Washington, DC, USA: 2007. Targeted Radionuclide Therapy.
-
- Berchuck A., Kamel A., Whitaker R., Kerns B., Olt G., Kinney R., Soper J.T., Dodge R., Clarke-Pearson D.L., Marks P., et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1991;50:4087–4091. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

