Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways
- PMID: 28536426
- PMCID: PMC5442131
- DOI: 10.1038/s41598-017-02347-2
Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways
Abstract
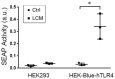
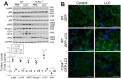
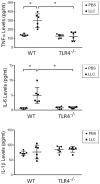
Cancer-induced cachexia, characterized by muscle wasting, is a lethal metabolic syndrome with undefined etiology. Current consensus is that multiple factors contribute to cancer-induced muscle wasting, and therefore therapy requires combinational strategies. Here, we show that Toll-like receptor 4 (TLR4) mediates cancer-induced muscle wasting by directly activating muscle catabolism as well as stimulating an innate immune response in mice bearing Lewis lung carcinoma (LLC), and targeting TLR4 alone effectively abrogate muscle wasting. Utilizing specific siRNAs we observed that LLC cell-conditioned medium (LCM)-treated C2C12 myotubes underwent a rapid catabolic response in a TLR4-dependent manner, including activation of the p38 MAPK-C/EBPβ signaling pathway as well as the ubiquitin-proteasome and autophagy-lysosome pathways, resulting in myotube atrophy. Utilizing a reporter cell-line it was confirmed that LCM activated TLR4. These results suggest that LLC-released cachexins directly activate muscle catabolism via activating TLR4 on muscle cells independent of immune responses. Critically, LLC tumor-bearing TLR4-/- mice were spared from muscle wasting due to a blockade in muscle catabolic pathways. Further, tumor-induced elevation of circulating TNFα and interleukin-6 (IL-6) was abolished in TLR4-/- mice. These data suggest that TLR4 is a central mediator and therapeutic target of cancer-induced muscle wasting.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Valproic acid attenuates skeletal muscle wasting by inhibiting C/EBPβ-regulated atrogin1 expression in cancer cachexia.Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C101-15. doi: 10.1152/ajpcell.00344.2015. Epub 2016 Apr 27. Am J Physiol Cell Physiol. 2016. PMID: 27122162
-
Cancer-Induced Muscle Wasting Requires p38β MAPK Activation of p300.Cancer Res. 2021 Feb 15;81(4):885-897. doi: 10.1158/0008-5472.CAN-19-3219. Epub 2020 Dec 22. Cancer Res. 2021. PMID: 33355181 Free PMC article.
-
C/EBPβ mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting.EMBO J. 2011 Aug 16;30(20):4323-35. doi: 10.1038/emboj.2011.292. EMBO J. 2011. PMID: 21847090 Free PMC article.
-
Apoptosis signalling is essential and precedes protein degradation in wasting skeletal muscle during catabolic conditions.Int J Biochem Cell Biol. 2008;40(9):1674-8. doi: 10.1016/j.biocel.2008.02.001. Epub 2008 Feb 14. Int J Biochem Cell Biol. 2008. PMID: 18329944 Review.
-
Cancer Takes a Toll on Skeletal Muscle by Releasing Heat Shock Proteins-An Emerging Mechanism of Cancer-Induced Cachexia.Cancers (Basel). 2019 Aug 30;11(9):1272. doi: 10.3390/cancers11091272. Cancers (Basel). 2019. PMID: 31480237 Free PMC article. Review.
Cited by
-
Impact of cancer cachexia on respiratory muscle function and the therapeutic potential of exercise.J Physiol. 2022 Dec;600(23):4979-5004. doi: 10.1113/JP283569. Epub 2022 Nov 1. J Physiol. 2022. PMID: 36251564 Free PMC article. Review.
-
Muscle alterations in the development and progression of cancer-induced muscle atrophy: a review.J Appl Physiol (1985). 2020 Jan 1;128(1):25-41. doi: 10.1152/japplphysiol.00622.2019. Epub 2019 Nov 14. J Appl Physiol (1985). 2020. PMID: 31725360 Free PMC article. Review.
-
Reporter Cell Assessment of TLR4-Induced NF-κB Responses to Cell-Free Hemoglobin and the Influence of Biliverdin.Biomedicines. 2019 Jun 3;7(2):41. doi: 10.3390/biomedicines7020041. Biomedicines. 2019. PMID: 31163699 Free PMC article.
-
Converged Rab37/IL-6 trafficking and STAT3/PD-1 transcription axes elicit an immunosuppressive lung tumor microenvironment.Theranostics. 2021 May 12;11(14):7029-7044. doi: 10.7150/thno.60040. eCollection 2021. Theranostics. 2021. PMID: 34093869 Free PMC article.
-
The myokine Fibcd1 is an endogenous determinant of myofiber size and mitigates cancer-induced myofiber atrophy.Nat Commun. 2022 May 2;13(1):2370. doi: 10.1038/s41467-022-30120-1. Nat Commun. 2022. PMID: 35501350 Free PMC article.
References
-
- Baracos VE, DeVivo C, Hoyle DH, Goldberg AL. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995;268:E996–1006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

