Circulating microRNA-214 and -126 as potential biomarkers for canine neoplastic disease
- PMID: 28536479
- PMCID: PMC5442106
- DOI: 10.1038/s41598-017-02607-1
Circulating microRNA-214 and -126 as potential biomarkers for canine neoplastic disease
Abstract
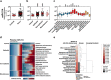
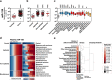
Circulating microRNAs in the blood may provide diagnostic and prognostic information about canine neoplastic diseases, and their profiles may be conserved between human and canine species. We performed RT-qPCR to obtain the profiles of circulating plasma microRNA-214 and -126 in total 181 cases of canine neoplastic diseases and healthy controls. MicroRNA-214 levels were high in 2 epithelial tumours (thyroid and mammary carcinomas) and 4 non-epithelial tumours (osteosarcoma, histiocytic sarcoma, chondrosarcoma, and hemangiosarcoma). In contrast, microRNA-126 levels were high in 6 epithelial tumours (mammary, hepatocellular, squamous cell, thyroid, transitional cell carcinomas, and adenocarcinoma) and 4 non-epithelial tumours (osteosarcoma, mast cell tumour, melanoma, and hemangiosarcoma). The diagnostic potential of microRNA-214 was relatively high in sarcomas, whereas that of microR-126 was high in most types of the tumours. MicroRNA-214 and -126 were prognostic predictors in 2 groups (adenocarcinoma and non-epithelial tumours except for osteosarcoma) and 3 groups (epithelial tumours, adenocarcinoma, and melanoma), respectively. Additionally, the microRNA levels did not show a strong correlation with the other clinical parameters. In conclusion, circulating microRNA-214 and -126 have the potential to be diagnostic and prognostic biomarkers for canine neoplastic diseases. Furthermore, their profiles may be key references as well for exploring novel biomarkers for human cancers.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
MicroRNA expression patterns in canine mammary cancer show significant differences between metastatic and non-metastatic tumours.BMC Cancer. 2017 Nov 7;17(1):728. doi: 10.1186/s12885-017-3751-1. BMC Cancer. 2017. PMID: 29115935 Free PMC article.
-
Circulating Pre-microRNA-488 in Peripheral Blood Is a Potential Biomarker for Predicting Recurrence in Breast Cancer.Anticancer Res. 2018 Aug;38(8):4515-4523. doi: 10.21873/anticanres.12755. Anticancer Res. 2018. PMID: 30061217
-
Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage.Clin Epigenetics. 2018 Apr 23;10:59. doi: 10.1186/s13148-018-0492-1. eCollection 2018. Clin Epigenetics. 2018. PMID: 29713393 Free PMC article. Review.
-
Detection of novel polymorphisms in the ckit gene of canine patients with lymphoma, melanoma, haemangiosarcoma, and osteosarcoma.Vet Res Commun. 2016 Jun;40(2):89-95. doi: 10.1007/s11259-016-9653-x. Epub 2016 Mar 12. Vet Res Commun. 2016. PMID: 26971271
-
Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers.Cell Death Dis. 2017 Sep 7;8(9):e3045. doi: 10.1038/cddis.2017.440. Cell Death Dis. 2017. PMID: 28880270 Free PMC article. Review.
Cited by
-
Identification of canine circulating miRNAs as tumor biospecific markers using Next-Generation Sequencing and Q-RT-PCR.Biochem Biophys Rep. 2021 Aug 19;28:101106. doi: 10.1016/j.bbrep.2021.101106. eCollection 2021 Dec. Biochem Biophys Rep. 2021. PMID: 34458596 Free PMC article.
-
MicroRNAs and angiosarcoma: are there promising reports?Front Oncol. 2024 May 17;14:1385632. doi: 10.3389/fonc.2024.1385632. eCollection 2024. Front Oncol. 2024. PMID: 38826780 Free PMC article. Review.
-
Low-Invasive Biomarkers of Canine Mammary Tumours.Vet Med Sci. 2025 Mar;11(2):e70280. doi: 10.1002/vms3.70280. Vet Med Sci. 2025. PMID: 40095734 Free PMC article. Review.
-
MicroRNAs as Biomarkers in Canine Osteosarcoma: A New Future?Vet Sci. 2020 Sep 30;7(4):146. doi: 10.3390/vetsci7040146. Vet Sci. 2020. PMID: 33008041 Free PMC article. Review.
-
Exploring the role of microRNAs as diagnostic and prognostic biomarkers in canine mammary tumors.Geroscience. 2024 Dec;46(6):6641-6657. doi: 10.1007/s11357-024-01260-7. Epub 2024 Jul 2. Geroscience. 2024. PMID: 38954129 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

