Helicobacter pylori antibody responses in association with eradication outcome and recurrence: a population-based intervention trial with 7.3-year follow-up in China
- PMID: 28536491
- PMCID: PMC5422414
- DOI: 10.21147/j.issn.1000-9604.2017.02.05
Helicobacter pylori antibody responses in association with eradication outcome and recurrence: a population-based intervention trial with 7.3-year follow-up in China
Abstract
Objective: To identify serum biomarkers that may predict the short or long term outcomes of anti-Helicobacter pylori (H. pylori) treatment, a follow-up study was performed based on an intervention trial in Linqu County, China.
Methods: A total of 529 subjects were selected randomly from 1,803 participants to evaluate total anti-H. pylori immunoglobulin G (IgG) and 10 specific antibody levels before and after treatment at 1-, 2- and 7.3-year. The outcomes of anti-H. pylori treatment were also parallelly assessed by13C-urea breath test at 45-d after treatment and 7.3-year at the end of follow-up.
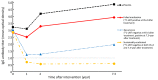
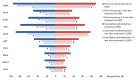
Results: We found the medians of anti-H. pylori IgG titers were consistently below cut-off value through 7.3 years in eradicated group, however, the medians declined in recurrence group to 1.2 at 1-year after treatment and slightly increased to 2.0 at 7.3-year. While the medians were significantly higher (>3.0 at 2- and 7.3-year) among subjects who failed the eradication or received placebo. For specific antibody responses, baseline seropositivities of FliD and HpaA were reversely associated with eradication failure [for FliD, odds ratio (OR)=0.44, 95% confidence interval (95% CI): 0.27-0.73; for HpaA, OR=0.32, 95% CI: 0.17-0.60]. The subjects with multiple positive specific antibodies at baseline were more likely to be successfully eradicated in a linear fashion (Ptrend=0.006).
Conclusions: Our study suggested that total anti-H. pylori IgG level may serve as a potential monitor of long-term impact on anti-H. pylori treatment, and priority forH. pylori treatment may be endowed to the subjects with multiple seropositive antibodies at baseline, especially for FliD and HapA.
Keywords: Helicobacter pylori; biomarker; recurrence; serology; treatment outcome.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
References
-
- Ohata H, Kitauchi S, Yoshimura N, , et al. Progression of chronic atrophic gastritis associated withHelicobacter pylori infection increases risk of gastric cancer . Int J Cancer. 2004;109:138–43. doi: 10.1002/ijc.11680. [Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated withHelicobacter pylori infection increases risk of gastric cancer. Int J Cancer 2004;109:138-43. <DOI: 10.1002/ijc.11680> <PMID: 14735480> ] - DOI - PubMed
-
- Helicobacter and Cancer Collaborative Group. Gastric cancer andHelicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts . Gut. 2001;49:347–53. [Helicobacter and Cancer Collaborative Group. Gastric cancer andHelicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001;49:347-53. <PMID: 11511555> ] - PMC - PubMed
-
- You WC, Brown LM, Zhang L, , et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J NatI Cancer Inst. 2006;98:974–83. doi: 10.1093/jnci/djj264. [You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J NatI Cancer Inst 2006;98:974-83. <DOI: 10.1093/jnci/djj264> <PMID: 16849680>] - DOI - PubMed
-
- Wong BC, Lam SK, Wong WM, , et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial . JAMA. 2004;291:187–94. doi: 10.1001/jama.291.2.187. [Wong BC, Lam SK, Wong WM, et al.Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187-94. <DOI: 10.1001/jama.291.2.187> <PMID: 14722144> ] - DOI - PubMed
-
- Fukase K, Kato M, Kikuchi S, , et al. Effect of eradication ofHelicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial . Lancet. 2008;372:392–7. doi: 10.1016/S0140-6736(08)61159-9. [Fukase K, Kato M, Kikuchi S, et al. Effect of eradication ofHelicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392-7. <DOI: 10.1016/S0140-6736(08)61159-9> <PMID: 18675689> ] - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
