Epigenetic Activation of ASCT2 in the Hippocampus Contributes to Depression-Like Behavior by Regulating D-Serine in Mice
- PMID: 28536503
- PMCID: PMC5422558
- DOI: 10.3389/fnmol.2017.00139
Epigenetic Activation of ASCT2 in the Hippocampus Contributes to Depression-Like Behavior by Regulating D-Serine in Mice
Abstract
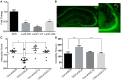
The roles of D-serine in depression are raised concerned recently as an intrinsic co-agonist for the NMDA receptor. However, the mechanisms underlying its regulation are not fully elucidated. ASCT2 is a Na+-dependent D-serine transporter. We found that decreased D-serine and increased hippocampal ASCT2 levels correlated with chronic social defeat stress (CSDS) in mice. Lentivirus-mediated shRNA-mediated knockdown of ASCT2 and the administration of exogenous D-serine in the hippocampus alleviated CSDS-induced social avoidance and immobility. In vivo and in vitro experiments revealed that upregulation of ASCT2 expression in CSDS was regulated through histone hyper-acetylation, not DNA methylation in its promoter region. Immunohistochemistry demonstrated the co-localization of ASCT2 and D-serine. Uptake of D-serine by ASCT2 was demonstrated by in vivo and in vitro experiments. Our results indicate that CSDS induces ASCT2 expression through epigenetic activation and decreases hippocampal D-serine levels, leading to social avoidance, and immobility. Thus, targeting D-serine transport represents an attractive new strategy for treating depression.
Keywords: ASCT2; D-serine; SLC1A5; chronic social defeat stress; depression; mouse.
Figures




Similar articles
-
D-Serine Is a Substrate for Neutral Amino Acid Transporters ASCT1/SLC1A4 and ASCT2/SLC1A5, and Is Transported by Both Subtypes in Rat Hippocampal Astrocyte Cultures.PLoS One. 2016 Jun 7;11(6):e0156551. doi: 10.1371/journal.pone.0156551. eCollection 2016. PLoS One. 2016. PMID: 27272177 Free PMC article.
-
Deletion of serine racemase confers D-serine -dependent resilience to chronic social defeat stress.Neurochem Int. 2018 Jun;116:43-51. doi: 10.1016/j.neuint.2018.03.008. Epub 2018 Mar 14. Neurochem Int. 2018. PMID: 29550603
-
Kososan, a Kampo medicine, prevents a social avoidance behavior and attenuates neuroinflammation in socially defeated mice.J Neuroinflammation. 2017 May 3;14(1):98. doi: 10.1186/s12974-017-0876-8. J Neuroinflammation. 2017. PMID: 28468634 Free PMC article.
-
The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology.Front Cell Dev Biol. 2018 Sep 4;6:96. doi: 10.3389/fcell.2018.00096. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30234109 Free PMC article. Review.
-
The role of ASCT2 in cancer: A review.Eur J Pharmacol. 2018 Oct 15;837:81-87. doi: 10.1016/j.ejphar.2018.07.007. Epub 2018 Jul 17. Eur J Pharmacol. 2018. PMID: 30025811 Review.
Cited by
-
Putative Inflammatory Sensitive Mechanisms Underlying Risk or Resilience to Social Stress.Front Behav Neurosci. 2018 Oct 26;12:240. doi: 10.3389/fnbeh.2018.00240. eCollection 2018. Front Behav Neurosci. 2018. PMID: 30416436 Free PMC article. Review.
-
Stachyose Alleviates Corticosterone-Induced Long-Term Potentiation Impairment via the Gut-Brain Axis.Front Pharmacol. 2022 Mar 10;13:799244. doi: 10.3389/fphar.2022.799244. eCollection 2022. Front Pharmacol. 2022. PMID: 35370743 Free PMC article.
-
D-Serine as the gatekeeper of NMDA receptor activity: implications for the pharmacologic management of anxiety disorders.Transl Psychiatry. 2020 Jun 9;10(1):184. doi: 10.1038/s41398-020-00870-x. Transl Psychiatry. 2020. PMID: 32518273 Free PMC article. Review.
-
Impeding the combination of astrocytic ASCT2 and NLRP3 by talniflumate alleviates neuroinflammation in experimental models of Parkinson's disease.Acta Pharm Sin B. 2023 Feb;13(2):662-677. doi: 10.1016/j.apsb.2022.07.021. Epub 2022 Aug 3. Acta Pharm Sin B. 2023. PMID: 36873178 Free PMC article.
-
Isorhynchophylline ameliorates stress-induced emotional disorder and cognitive impairment with modulation of NMDA receptors.Front Neurosci. 2022 Dec 15;16:1071068. doi: 10.3389/fnins.2022.1071068. eCollection 2022. Front Neurosci. 2022. PMID: 36590289 Free PMC article.
References
-
- Bagot R. C., Zhang T. Y., Wen X., Nguyen T. T., Nguyen H. B., Diorio J., et al. . (2012). Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proc. Natl. Acad. Sci. U.S.A. 109(Suppl. 2), 17200–17207. 10.1073/pnas.1204599109 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

