Thyroid Hormone Induces PGC-1α during Dendritic Outgrowth in Mouse Cerebellar Purkinje Cells
- PMID: 28536504
- PMCID: PMC5422430
- DOI: 10.3389/fncel.2017.00133
Thyroid Hormone Induces PGC-1α during Dendritic Outgrowth in Mouse Cerebellar Purkinje Cells
Abstract
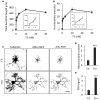
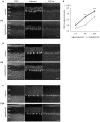
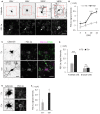
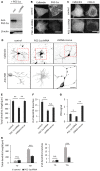
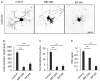
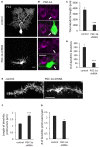
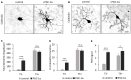
Thyroid hormone 3,3',5-Triiodo-L-thyronine (T3) is essential for proper brain development. Perinatal loss of T3 causes severe growth defects in neurons and glia, including strong inhibition of dendrite formation in Purkinje cells in the cerebellar cortex. Here we show that T3 promotes dendritic outgrowth of Purkinje cells through induction of peroxisome proliferator-activated receptor gamma (PPARγ) co-activator 1α (PGC-1α), a master regulator of mitochondrial biogenesis. PGC-1α expression in Purkinje cells is upregulated during dendritic outgrowth in normal mice, while it is significantly retarded in hypothyroid mice or in cultures depleted of T3. In cultured Purkinje cells, PGC-1α knockdown or molecular perturbation of PGC-1α signaling inhibits enhanced dendritic outgrowth and mitochondrial generation and activation caused by T3 treatment. In contrast, PGC-1α overexpression promotes dendrite extension even in the absence of T3. PGC-1α knockdown also downregulates dendrite formation in Purkinje cells in vivo. Our findings suggest that the growth-promoting activity of T3 is partly mediated by PGC-1α signaling in developing Purkinje cells.
Keywords: PGC-1α; Purkinje cell; dendritogenesis; hypothyroid; mitochondria; thyroid hormone.
Figures

Similar articles
-
Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor alpha1.J Neurosci. 2003 Nov 19;23(33):10604-12. doi: 10.1523/JNEUROSCI.23-33-10604.2003. J Neurosci. 2003. PMID: 14627645 Free PMC article.
-
Thyroid hormone activation by type 2 deiodinase mediates exercise-induced peroxisome proliferator-activated receptor-γ coactivator-1α expression in skeletal muscle.J Physiol. 2016 Sep 15;594(18):5255-69. doi: 10.1113/JP272440. Epub 2016 Aug 18. J Physiol. 2016. PMID: 27302464 Free PMC article.
-
T3-mediated expression of PGC-1alpha via a far upstream located thyroid hormone response element.Mol Cell Endocrinol. 2008 Jun 11;287(1-2):90-5. doi: 10.1016/j.mce.2008.01.017. Epub 2008 Feb 3. Mol Cell Endocrinol. 2008. PMID: 18336995
-
T3-mediated gene expression is independent of PGC-1alpha.Mol Cell Endocrinol. 2007 May 30;270(1-2):57-63. doi: 10.1016/j.mce.2007.02.008. Epub 2007 Feb 24. Mol Cell Endocrinol. 2007. PMID: 17382463
-
Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α.Arthritis Rheumatol. 2015 May;67(8):2141-53. doi: 10.1002/art.39182. Arthritis Rheumatol. 2015. PMID: 25940958 Free PMC article.
Cited by
-
Role of irisin in Chinese patients with hypothyroidism: an interventional study.J Int Med Res. 2019 Apr;47(4):1592-1601. doi: 10.1177/0300060518824445. Epub 2019 Feb 6. J Int Med Res. 2019. PMID: 30722716 Free PMC article.
-
Involvement of integrin αvβ3 in thyroid hormone-induced dendritogenesis.Front Endocrinol (Lausanne). 2022 Aug 22;13:938596. doi: 10.3389/fendo.2022.938596. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36072926 Free PMC article.
-
Scrap and Build for Functional Neural Circuits: Spatiotemporal Regulation of Dendrite Degeneration and Regeneration in Neural Development and Disease.Front Cell Neurosci. 2021 Jan 11;14:613320. doi: 10.3389/fncel.2020.613320. eCollection 2020. Front Cell Neurosci. 2021. PMID: 33505249 Free PMC article. Review.
-
Thyroid hormone modulates hyperoxic neonatal lung injury and mitochondrial function.JCI Insight. 2023 Apr 24;8(8):e160697. doi: 10.1172/jci.insight.160697. JCI Insight. 2023. PMID: 36917181 Free PMC article.
-
3,3'-Dichlorobiphenyl (PCB 11) promotes dendritic arborization in primary rat cortical neurons via a CREB-dependent mechanism.Arch Toxicol. 2018 Nov;92(11):3337-3345. doi: 10.1007/s00204-018-2307-8. Epub 2018 Sep 17. Arch Toxicol. 2018. PMID: 30225637 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

