BAG2 structure, function and involvement in disease
- PMID: 28536620
- PMCID: PMC5415834
- DOI: 10.1186/s11658-016-0020-2
BAG2 structure, function and involvement in disease
Abstract
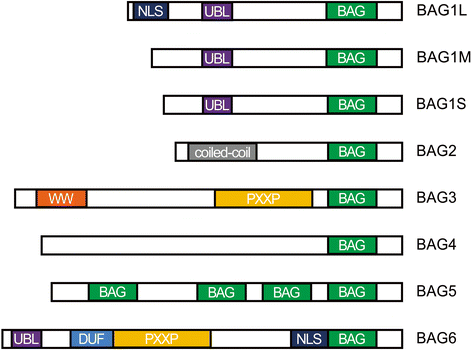
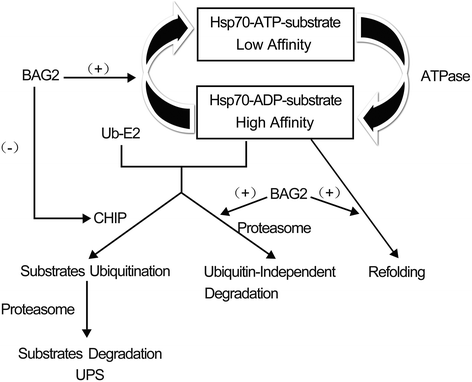
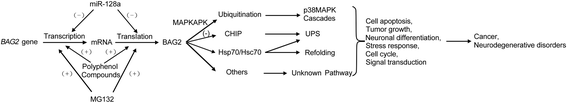
Bcl2-associated athanogene 2 (BAG2) shares a similar molecular structure and function with other BAG family members. Functioning as a co-chaperone, it interacts with the ATPase domain of the heat shock protein 70 (dHsp70) through its BAG domain. It also interacts with many other molecules and regulates various cellular functions. An increasing number of studies have indicated that BAG2 is involved in the pathogenesis of various diseases, including cancers and neurodegenerative diseases. This paper is a comprehensive review of the structure, functions, and protein interactions of BAG2. We also discuss its roles in diseases, including cancer, Alzheimer's disease, Parkinson's disease and spinocerebellar ataxia type-3. Further research on BAG2 could lead to an understanding of the pathogenesis of these disorders or even to novel therapeutic approaches.
Keywords: Alzheimer’s disease; BAG2; Carcinoma; Molecular chaperones; Parkinson’s disease; Spinocerebellar ataxia type-3.
Figures
Similar articles
-
Comparative analysis of BAG1 and BAG2: Insights into their structures, functions and implications in disease pathogenesis.Int Immunopharmacol. 2024 Dec 25;143(Pt 1):113369. doi: 10.1016/j.intimp.2024.113369. Epub 2024 Oct 14. Int Immunopharmacol. 2024. PMID: 39405938 Review.
-
The BAG2 and BAG5 proteins inhibit the ubiquitination of pathogenic ataxin3-80Q.Int J Neurosci. 2015 May;125(5):390-4. doi: 10.3109/00207454.2014.940585. Epub 2014 Aug 19. Int J Neurosci. 2015. PMID: 25006867
-
Protein-misfolding diseases and chaperone-based therapeutic approaches.FEBS J. 2006 Apr;273(7):1331-49. doi: 10.1111/j.1742-4658.2006.05181.x. FEBS J. 2006. PMID: 16689923 Review.
-
Targeting Chaperone/Co-Chaperone Interactions with Small Molecules: A Novel Approach to Tackle Neurodegenerative Diseases.Cells. 2021 Sep 29;10(10):2596. doi: 10.3390/cells10102596. Cells. 2021. PMID: 34685574 Free PMC article. Review.
-
Modulation of neurodegeneration by molecular chaperones.Nat Rev Neurosci. 2005 Jan;6(1):11-22. doi: 10.1038/nrn1587. Nat Rev Neurosci. 2005. PMID: 15611723 Review.
Cited by
-
Chlamydia pneumoniae can infect the central nervous system via the olfactory and trigeminal nerves and contributes to Alzheimer's disease risk.Sci Rep. 2022 Feb 17;12(1):2759. doi: 10.1038/s41598-022-06749-9. Sci Rep. 2022. PMID: 35177758 Free PMC article.
-
Comparative Multiplexed Interactomics of SARS-CoV-2 and Homologous Coronavirus Nonstructural Proteins Identifies Unique and Shared Host-Cell Dependencies.ACS Infect Dis. 2020 Dec 11;6(12):3174-3189. doi: 10.1021/acsinfecdis.0c00500. Epub 2020 Dec 2. ACS Infect Dis. 2020. PMID: 33263384 Free PMC article.
-
Dynamic Intestinal Stem Cell Plasticity and Lineage Remodeling by a Nutritional Environment Relevant to Human Risk for Tumorigenesis.Mol Cancer Res. 2023 Aug 1;21(8):808-824. doi: 10.1158/1541-7786.MCR-22-1000. Mol Cancer Res. 2023. PMID: 37097719 Free PMC article.
-
Muscle Proteomic Profile before and after Enzyme Replacement Therapy in Late-Onset Pompe Disease.Int J Mol Sci. 2021 Mar 11;22(6):2850. doi: 10.3390/ijms22062850. Int J Mol Sci. 2021. PMID: 33799647 Free PMC article.
-
A proximity labeling strategy enables proteomic analysis of inter-organelle membrane contacts.iScience. 2023 Jun 17;26(7):107159. doi: 10.1016/j.isci.2023.107159. eCollection 2023 Jul 21. iScience. 2023. PMID: 37485370 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

