PPARγ agonist through the terminal differentiation phase is essential for adipogenic differentiation of fetal ovine preadipocytes
- PMID: 28536637
- PMCID: PMC5415806
- DOI: 10.1186/s11658-017-0037-1
PPARγ agonist through the terminal differentiation phase is essential for adipogenic differentiation of fetal ovine preadipocytes
Abstract
Background: Although the 3T3-L1 preadipocyte cell line represents an informative model for in vitro adipogenesis research, primary cultured cells are often needed to understand particular human or animal metabolic phenotypes. As demonstrated by in vitro cultured preadipocytes from large mammalian species, primary cultured cells require specific adipogenic differentiation conditions different to that of the 3T3-L1 cell line. These conditions are also species-specific and require optimization steps. However, efficient protocols to differentiate primary preadipocytes using alternative species to rodents are scarce. Sheep represent an amenable animal model for fetal biology and developmental origins of health and disease studies. In this work, we present with the first detailed procedure to efficiently differentiate primary fetal and adult ovine preadipocytes.
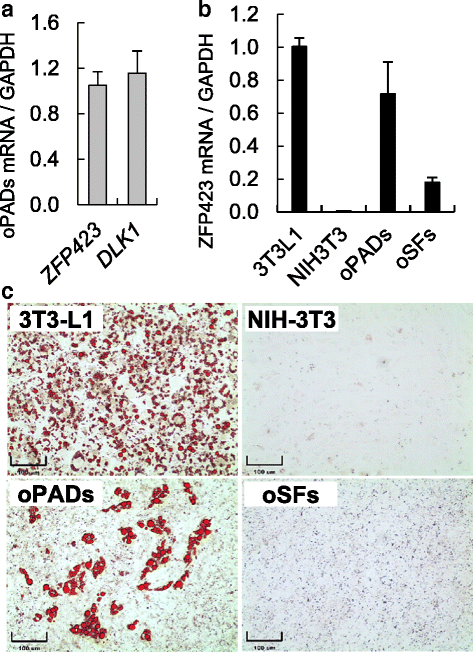
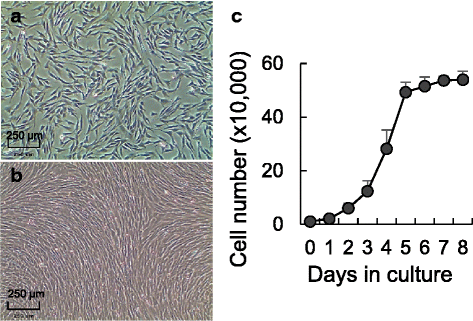
Methods: Fetal and adult ovine adipose and skin tissue harvest, preadipocyte and fibroblast isolation, proliferation, and standardization and optimization of a new adipogenic differentiation protocol. Use of commercial cell lines (3T3-L1 and NIH-3T3) for validation purposes. Oil red O stain and gene expression were used to validate adipogenic differentiation. ANOVA and Fisher's exact test were used to determine statistical significance.
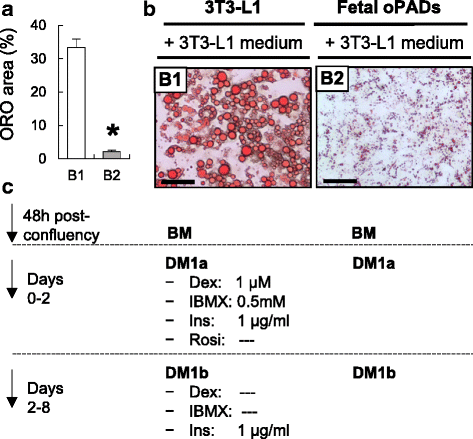
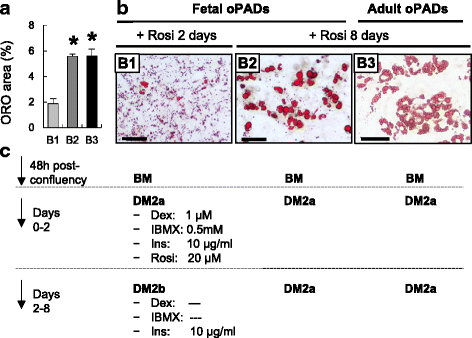
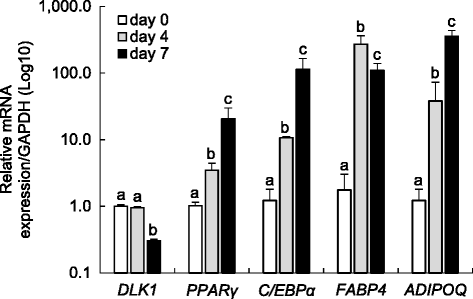
Results: Our optimized adipogenic differentiation method included a prolonged adipogenic cocktail exposure time from 2 to 8 days, higher insulin concentration, and supplementation with the peroxisome proliferator-activated receptor gamma (PPARγ) agonist, rosiglitazone. This protocol was optimized for both, fetal and adult preadipocytes.
Conclusions: Our protocol enables successful adipogenic differentiation of fetal and adult ovine preadipocytes. This work demonstrates that compared to the 3T3-L1 cell line, fetal ovine preadipocytes require a longer exposure to the differentiation cocktail, and the need for IMBX, dexamethasone, and/or the PPARγ agonist rosiglitazone through the terminal differentiation phase. They also require higher insulin concentration during differentiation to enhance lipid accumulation and similar to human primary preadipocytes, PPARγ agonist supplementation is also required for ovine adipogenic differentiation. This work highlights species-specific differences requirements for adipogenic differentiation and the need to develop standardized methods to investigate comparative adipocyte biology.
Keywords: Adipogenic differentiation; Fetal; Preadipocyte; Sheep.
Figures
References
-
- Benyshek DC. The “early life” origins of obesity-related health disorders: new discoveries regarding the intergenerational transmission of developmentally programmed traits in the global cardiometabolic health crisis. Am J Phys Anthropol. 2013 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

