Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck
- PMID: 28536670
- PMCID: PMC5422557
- DOI: 10.3389/fonc.2017.00072
Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck
Abstract
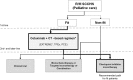
The major development of the past decade in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) was the introduction of cetuximab in combination with platinum plus 5-fluorouracil chemotherapy (CT), followed by maintenance cetuximab (the "EXTREME" regimen). This regimen is supported by a phase 3 randomized trial and subsequent observational studies, and it confers well-documented survival benefits, with median survival ranging between approximately 10 and 14 months, overall response rates between 36 and 44%, and disease control rates of over 80%. Furthermore, as indicated by patient-reported outcome measures, the addition of cetuximab to platinum-based CT leads to a significant reduction in pain and problems with social eating and speech. Conversely, until very recently, there has been a lack of evidence-based second-line treatment options, and the therapies that have been available have shown low response rates and poor survival outcomes. Presently, a promising new treatment option in R/M SCCHN has emerged: immune checkpoint inhibitors (ICIs), which have demonstrated favorable results in second-line clinical trials. Nivolumab and pembrolizumab are the first two ICIs that were approved by the US Food and Drug Administration. We note that the trials that showed benefit with ICIs included not only patients who previously received ≥1 platinum-based regimens for R/M SCCHN but also patients who experienced recurrence within 6 months after combined modality therapy with a platinum agent for locally advanced disease. In this review, we outline the available clinical and observational evidence for the EXTREME regimen and the initial results from clinical trials for ICIs in patients with R/M SCCHN. We propose that these treatment options can be integrated into a new continuum of care paradigm, with first-line EXTREME regimen followed by second-line ICIs. A number of ongoing clinical trials are comparing regimens with ICIs, alone and in combination with other ICIs or CT, with the EXTREME regimen for first-line treatment of R/M SCCHN. As we eagerly await the results of these trials, the EXTREME regimen remains the standard of care for the first-line treatment of R/M SCCHN.
Keywords: EXTREME; cetuximab; immune checkpoint inhibitor; platinum-refractory; programmed cell death ligand 1; programmed cell death protein 1; recurrent and/or metastatic; squamous cell carcinoma of the head and neck.
Figures
References
-
- GLOBCAN. GLOBCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. World Health Organization, International Agency for Research on Cancer Web site; (2012). Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
-
- Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortés-Funes H, Hitt R, et al. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol (2005) 23(24):5568–77. 10.1200/JCO.2005.07.119 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

