An Overview of Two-Component Signal Transduction Systems Implicated in Extra-Intestinal Pathogenic E. coli Infections
- PMID: 28536675
- PMCID: PMC5422438
- DOI: 10.3389/fcimb.2017.00162
An Overview of Two-Component Signal Transduction Systems Implicated in Extra-Intestinal Pathogenic E. coli Infections
Abstract
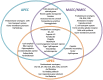
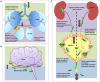
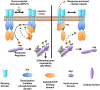
Extra-intestinal pathogenic E. coli (ExPEC) infections are common in mammals and birds. The predominant ExPEC types are avian pathogenic E. coli (APEC), neonatal meningitis causing E. coli/meningitis associated E. coli (NMEC/MAEC), and uropathogenic E. coli (UPEC). Many reviews have described current knowledge on ExPEC infection strategies and virulence factors, especially for UPEC. However, surprisingly little has been reported on the regulatory modules that have been identified as critical in ExPEC pathogenesis. Two-component systems (TCSs) comprise the predominant method by which bacteria respond to changing environments and play significant roles in modulating bacterial fitness in diverse niches. Recent studies have highlighted the potential of manipulating signal transduction systems as a means to chemically re-wire bacterial pathogens, thereby reducing selective pressure and avoiding the emergence of antibiotic resistance. This review begins by providing a brief introduction to characterized infection strategies and common virulence factors among APEC, NMEC, and UPEC and continues with a comprehensive overview of two-component signal transduction networks that have been shown to influence ExPEC pathogenesis.
Keywords: APEC; ExPEC; MAEC/NMEC; UPEC; signal transduction; two-component systems; virulence factors.
Figures

Similar articles
-
Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: how closely related are they?Int J Med Microbiol. 2007 Jun;297(3):163-76. doi: 10.1016/j.ijmm.2007.01.003. Epub 2007 Mar 19. Int J Med Microbiol. 2007. PMID: 17374506
-
High frequency of hybrid Escherichia coli strains with combined Intestinal Pathogenic Escherichia coli (IPEC) and Extraintestinal Pathogenic Escherichia coli (ExPEC) virulence factors isolated from human faecal samples.BMC Infect Dis. 2018 Nov 1;18(1):544. doi: 10.1186/s12879-018-3449-2. BMC Infect Dis. 2018. PMID: 30497396 Free PMC article.
-
Zoonotic potential of Escherichia coli isolates from retail chicken meat products and eggs.Appl Environ Microbiol. 2015 Feb;81(3):1177-87. doi: 10.1128/AEM.03524-14. Epub 2014 Dec 5. Appl Environ Microbiol. 2015. PMID: 25480753 Free PMC article.
-
Extra-intestinal pathogenic Escherichia coli (ExPEC): Disease, carriage and clones.J Infect. 2015 Dec;71(6):615-26. doi: 10.1016/j.jinf.2015.09.009. Epub 2015 Sep 26. J Infect. 2015. PMID: 26409905 Review.
-
Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli.FEMS Immunol Med Microbiol. 2011 Jun;62(1):1-10. doi: 10.1111/j.1574-695X.2011.00797.x. Epub 2011 Mar 24. FEMS Immunol Med Microbiol. 2011. PMID: 21362060 Review.
Cited by
-
Virulence determinants and antimicrobial resistance of E. coli isolated from bovine clinical mastitis in some selected dairy farms of Bangladesh.Saudi J Biol Sci. 2021 Nov;28(11):6317-6323. doi: 10.1016/j.sjbs.2021.06.099. Epub 2021 Jul 6. Saudi J Biol Sci. 2021. PMID: 34759751 Free PMC article.
-
Responses of Escherichia coli and Listeria monocytogenes to ozone treatment on non-host tomato: Efficacy of intervention and evidence of induced acclimation.PLoS One. 2021 Oct 28;16(10):e0256324. doi: 10.1371/journal.pone.0256324. eCollection 2021. PLoS One. 2021. PMID: 34710139 Free PMC article.
-
Xenobiotic Effects of Chlorine Dioxide to Escherichia coli O157:H7 on Non-host Tomato Environment Revealed by Transcriptional Network Modeling: Implications to Adaptation and Selection.Front Microbiol. 2020 Jun 3;11:1122. doi: 10.3389/fmicb.2020.01122. eCollection 2020. Front Microbiol. 2020. PMID: 32582084 Free PMC article.
-
Transcriptome profiling of avian pathogenic Escherichia coli and the mouse microvascular endothelial cell line bEnd.3 during interaction.PeerJ. 2020 May 21;8:e9172. doi: 10.7717/peerj.9172. eCollection 2020. PeerJ. 2020. PMID: 32509459 Free PMC article.
-
Interaction of lipoprotein QseG with sensor kinase QseE in the periplasm controls the phosphorylation state of the two-component system QseE/QseF in Escherichia coli.PLoS Genet. 2018 Jul 24;14(7):e1007547. doi: 10.1371/journal.pgen.1007547. eCollection 2018 Jul. PLoS Genet. 2018. PMID: 30040820 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

