Improving Medication Adherence with Two-way Short Message Service Reminders in Sickle Cell Disease and Asthma. A feasibility randomized controlled trial
- PMID: 28536723
- PMCID: PMC6241750
- DOI: 10.4338/ACI-2016-12-RA-0203
Improving Medication Adherence with Two-way Short Message Service Reminders in Sickle Cell Disease and Asthma. A feasibility randomized controlled trial
Abstract
Introduction: Sickle cell disease (SCD) is a childhood and adult disease that primarily affects African Americans, characterized by life threatening sequelae mitigated by medications. One-way and two-way short message service (SMS) medication reminders have differing efficacy in chronic diseases. There is limited literature about SMS medication reminders in SCD.
Objective: The goal of this study was to test the feasibility, defined by recruitment/acceptance, retention/attrition, and technology utilization, of two-way SMS medication reminders in individuals with SCD with and without asthma.
Materials and methods: Participants were randomly allocated to standard care or reminders. Two-way SMS reminders were automated using Research Electronic Data Capture (REDCap) for hydroxyurea, fluticasone, budesonide and montelukast. Adherence was measured using the Morisky Medication Adherence Scale-8 (MMAS-8). Asthma control was assessed using the Childhood and Adult-Asthma Control Tests (ACT). Participants were enrolled 28 to 60 days with a common termination date.
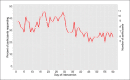
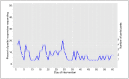
Results: The recruitment rate was 95% (47/49) and 82.9% completed the study. Among the 47 study participants enrolled, 51.1% were male, 61.7% were adults, median age was 20 (range: 3 to 59), and 98% were African Americans. Of the 26 participants receiving messages, 20% responded on over 95% of the days and usage varied with an average response rate of 33%, ranging from 21% to 46%. Medication adherence scores improved significantly in the intervention group (3.42 before, 5.46 after; p=0.002), but not in the control group (3.90 before, 4.75 after; p=0.080). Childhood-ACT scores improved in the intervention group (19.20 before, 24.25 after). Adult-ACT scores within the intervention arm were unchanged (21.0 before, 22.0 after. ACT scores did not improve significantly.
Conclusion: This study demonstrated the feasibility for two-way SMS medication reminders to improve medication adherence in a high-risk population where daily medication adherence is critical to health outcomes and quality of life.
Keywords: Sickle cell anemia; asthma; medication adherence; short message service; text.
Conflict of interest statement
Figures




Similar articles
-
The feasibility of text reminders to improve medication adherence in adolescents with asthma.J Am Med Inform Assoc. 2016 May;23(3):449-55. doi: 10.1093/jamia/ocv158. Epub 2015 Dec 11. J Am Med Inform Assoc. 2016. PMID: 26661717 Free PMC article. Clinical Trial.
-
A Multidimensional Electronic Hydroxyurea Adherence Intervention for Children With Sickle Cell Disease: Single-Arm Before-After Study.JMIR Mhealth Uhealth. 2019 Aug 8;7(8):e13452. doi: 10.2196/13452. JMIR Mhealth Uhealth. 2019. PMID: 31397291 Free PMC article.
-
Controller adherence following hospital discharge in high risk children: A pilot randomized trial of text message reminders.J Asthma. 2019 Jan;56(1):95-103. doi: 10.1080/02770903.2018.1424195. Epub 2018 Feb 13. J Asthma. 2019. PMID: 29437489 Free PMC article. Clinical Trial.
-
Mobile Telephone Text Messaging for Medication Adherence in Chronic Disease: A Meta-analysis.JAMA Intern Med. 2016 Mar;176(3):340-9. doi: 10.1001/jamainternmed.2015.7667. JAMA Intern Med. 2016. PMID: 26831740 Review.
-
Effectiveness of mobile phone text message reminder interventions to improve adherence to antiretroviral therapy among adolescents living with HIV: A systematic review and meta-analysis.PLoS One. 2021 Jul 22;16(7):e0254890. doi: 10.1371/journal.pone.0254890. eCollection 2021. PLoS One. 2021. PMID: 34293033 Free PMC article.
Cited by
-
Digital interventions to improve adherence to maintenance medication in asthma.Cochrane Database Syst Rev. 2022 Jun 13;6(6):CD013030. doi: 10.1002/14651858.CD013030.pub2. Cochrane Database Syst Rev. 2022. PMID: 35691614 Free PMC article.
-
Clinicians' Values and Preferences for Medication Adherence and Cost Clinical Decision Support in Primary Care: A Qualitative Study.Appl Clin Inform. 2020 May;11(3):405-414. doi: 10.1055/s-0040-1712467. Epub 2020 Jun 3. Appl Clin Inform. 2020. PMID: 32492717 Free PMC article.
-
Self-Reported Medication Adherence Measured with Morisky Scales in Rare Disease Patients: A Systematic Review and Meta-Analysis.Healthcare (Basel). 2023 May 31;11(11):1609. doi: 10.3390/healthcare11111609. Healthcare (Basel). 2023. PMID: 37297749 Free PMC article. Review.
-
Randomized pilot trial of cell phone support to improve medication adherence among adolescents and young adults with chronic health conditions.BMC Digit Health. 2024;2(1):13. doi: 10.1186/s44247-024-00069-w. Epub 2024 Mar 19. BMC Digit Health. 2024. PMID: 39211575 Free PMC article.
-
A Clinically Integrated mHealth App and Practice Model for Collecting Patient-Reported Outcomes between Visits for Asthma Patients: Implementation and Feasibility.Appl Clin Inform. 2019 Oct;10(5):783-793. doi: 10.1055/s-0039-1697597. Epub 2019 Oct 16. Appl Clin Inform. 2019. PMID: 31618782 Free PMC article.
References
-
- Prevention CfDCa. Data & Statistics 2015 [cited 2015 December 10]. Available from:http://www.cdc.gov/ncbddd/sicklecell/data.html
-
- Fox S.Health Topics: @pewinternet 2011[cited 2015 6/14/2015]. Available from:http://www.pewinternet.org/2011/02/01/health-topics-2/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

