Cytoprotective propensity of green tea polyphenols against citrinin-induced skeletal-myotube damage in C2C12 cells
- PMID: 28536872
- PMCID: PMC5507847
- DOI: 10.1007/s10616-017-0077-4
Cytoprotective propensity of green tea polyphenols against citrinin-induced skeletal-myotube damage in C2C12 cells
Abstract
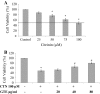
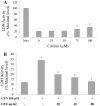
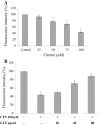
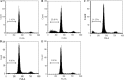
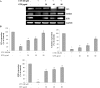
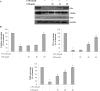
The mycotoxin citrinin, is produced by several species of Penicillium, Aspergillus and Monascus, and is capable of inducing cytotoxicity, oxidative stress and apoptosis. The aim of the present study was to investigate the effect of citrinin in mouse skeletal muscle cells (C2C12) and to overcome the cellular adverse effects by supplementing green tea extract (GTE) rich in polyphenols. C2C12 myoblasts were differentiated to myotubes and were exposed to citrinin in a dose dependent manner (0-100 µM) for 24 h and IC50 value was found to be 100 µM that resulted in decreased cell viability, increased LDH leakage and compromised membrane integrity. Mitochondrial membrane potential loss, increased accumulation of intracellular ROS and sub G1 phase of cell cycle was observed. To ameliorate the cytotoxic effects of CTN, C2C12 cells were pretreated with GTE (20, 40, 80 µg/ml) for 2 h followed by citrinin (100 µM) treatment for 24 h. GTE pretreatment combated citrinin-induced cytotoxicity and oxidative stress. GTE at 40 and 80 µg/ml significantly promoted cell survival and upregulated antioxidant enzyme activities (CAT, SOD, GPx) and endogenous antioxidant GSH, while the gene and protein expression levels were significantly restored through its effective antioxidant mechanism. Present study results suggested the antioxidant properties of GTE as a herbal source in ameliorating the citrinin-induced oxidative stress.
Keywords: C2C12 cells; Citrinin; Cytotoxicity; Green tea polyphenols; Lactate dehydrogenase; Oxidative stress.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures




Similar articles
-
Eugenol protects against citrinin-induced cytotoxicity and oxidative damages in cultured human colorectal HCT116 cells.Environ Sci Pollut Res Int. 2019 Oct;26(30):31374-31383. doi: 10.1007/s11356-019-06212-9. Epub 2019 Aug 31. Environ Sci Pollut Res Int. 2019. PMID: 31473926
-
Pelargonidin Modulates Keap1/Nrf2 Pathway Gene Expression and Ameliorates Citrinin-Induced Oxidative Stress in HepG2 Cells.Front Pharmacol. 2017 Nov 27;8:868. doi: 10.3389/fphar.2017.00868. eCollection 2017. Front Pharmacol. 2017. PMID: 29230174 Free PMC article.
-
Citrinin-generated reactive oxygen species cause cell cycle arrest leading to apoptosis via the intrinsic mitochondrial pathway in mouse skin.Toxicol Sci. 2011 Aug;122(2):557-66. doi: 10.1093/toxsci/kfr143. Epub 2011 May 27. Toxicol Sci. 2011. PMID: 21622943
-
The Effect of Green Tea and Sour Tea (Hibiscus sabdariffa L.) Supplementation on Oxidative Stress and Muscle Damage in Athletes.J Diet Suppl. 2017 May 4;14(3):346-357. doi: 10.1080/19390211.2016.1237400. Epub 2016 Oct 13. J Diet Suppl. 2017. PMID: 27736246 Clinical Trial.
-
Toxicological properties of citrinin.Arh Hig Rada Toksikol. 2009 Dec;60(4):457-64. doi: 10.2478/10004-1254-60-2009-1992. Arh Hig Rada Toksikol. 2009. PMID: 20061247 Review.
Cited by
-
Effects of tannase-converted green tea extract on skeletal muscle development.BMC Complement Med Ther. 2020 Feb 11;20(1):47. doi: 10.1186/s12906-020-2827-7. BMC Complement Med Ther. 2020. PMID: 32046706 Free PMC article.
-
Impacts of Green Tea on Joint and Skeletal Muscle Health: Prospects of Translational Nutrition.Antioxidants (Basel). 2020 Oct 28;9(11):1050. doi: 10.3390/antiox9111050. Antioxidants (Basel). 2020. PMID: 33126483 Free PMC article. Review.
-
Effect of Monascus-fermented Moringa oleifera on production performance, carcass characteristics, and meat quality attributes in broilers.Poult Sci. 2024 Dec;103(12):104306. doi: 10.1016/j.psj.2024.104306. Epub 2024 Sep 6. Poult Sci. 2024. PMID: 39303353 Free PMC article.
-
Effect of Spirulina and Fish Processing By-Products Extracts on Citrinin-Induced Cytotoxicity in SH-SY5Y Cells.Foods. 2024 Jun 19;13(12):1932. doi: 10.3390/foods13121932. Foods. 2024. PMID: 38928871 Free PMC article.
-
Differential Effects of Green Tea Powders on the Protection of Brown Tsaiya and Kaiya Ducklings against Trichothecene T-2 Toxin Toxicity.Animals (Basel). 2021 Aug 30;11(9):2541. doi: 10.3390/ani11092541. Animals (Basel). 2021. PMID: 34573507 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

